
��Ƴ���ͭ��ˮ�к���CN-��Cr2O72- ���ӣ���Ҫ������������ŷš��ó��ⶨ�������̽��з�ˮ�������ش��������⣺

��1������������ˮ��������Ҫʹ�õķ�����_________________��
��2�����з�Ӧ��������ų����÷�Ӧ�����ӷ���ʽΪ______________��
��3��������У�ÿ����0.4mol Cr2O72-ʱת�Ƶ���2.4mol���÷�Ӧ�����ӷ���ʽΪ ��
��4��ȡ��������ˮ�����Թ��У�����NaOH��Һ���۲쵽����ɫ�������ɣ��ټ�Na2S��Һ����ɫ����ת���ɺ�ɫ��������ʹ�û�ѧ��������ֽ��Ͳ����������ԭ�� ��
��5��Ŀǰ��������Cr2O72-��ˮ������������巨���÷������ˮ�м���FeSO4 ��7H2O��Cr2O72-��ԭ��Cr3+������pH��Fe��Crת�����൱�ڣ� ����������,�������ֱ�ʾԪ�ؼ�̬���ij�����
����������,�������ֱ�ʾԪ�ؼ�̬���ij�����
����1mol Cr2O72-�������a mol FeSO4 • 7H2O�����н�����ȷ����_______��
A. x =0.5 ,a =8 B. x =0.5 ,a = 10 C. x = 1.5 ,a =8 D. x = 1.5 ,a = 10
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ɫ��Ӧʵ���õIJ�˿��ÿ����һ����Ʒ����
A����ˮϴ��2��3�κ���ʹ��
B��������ϴ�Ӻ�����ˮ��ϴ������ʹ��
C������ֽ���ɺ�ſ�ʹ��
D��������ϴ�Ӻ����ھƾ��ƻ��������յ�û����ɫ���ſ�ʹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ɫ��Ӧʵ���õIJ�˿��ÿ����һ����Ʒ����
A����ˮϴ��2��3�κ���ʹ��
B��������ϴ�Ӻ�����ˮ��ϴ������ʹ��
C������ֽ���ɺ�ſ�ʹ��
D��������ϴ�Ӻ����ھƾ��ƻ��������յ�û����ɫ���ſ�ʹ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������һ�ֵ��͵�ǿ��������������ʵ���һ����ڻ��������ж�����Ҫ��Ӧ�á�
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���
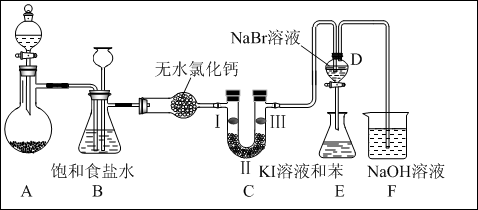
��1���Ʊ�����ѡ�õ�ҩƷΪ��������غ�Ũ���ᣬ��Ӧ�����ӷ���ʽΪ�� ��
��2��װ��B�������� �����ʵ�����ʱC�п��ܷ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ����ѡa��b��c��
| a | b | c | |
| I | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | Ũ���� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����塣��������D�е�������Һ����E�У���E���۲쵽�������� �������� ����ܡ����ܡ���˵����ķǽ�����ǿ�ڵ⣬ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ�о���ѧϰС��Ե������Һ�����µĹ����ܽᣨ���ڳ����£���������ȷ����
�� pH��1��ǿ����Һ����ˮϡ�ͺ���Һ�и�����Ũ�ȶ��ή��
�� 1 L 0.50 mol��L��1NH4Cl ��Һ��2 L 0.25 mol��L��1NH4Cl ��Һ��NH4+ ���ʵ�����ȫ���
�� pH��ȵ�������Һ��a��CH3COONa b��C6H5ONa c��NaHCO3 d��NaOH����������Һ�����ʵ����ʵ���Ũ����С����˳��Ϊ��d < b < c < a
�� pH=8.3��NaHCO3��Һ��c(Na��) �� c(HCO3��) �� c(CO32��)�� c(H2CO3)
�� pH��2��һԪ���pH��12�Ķ�Ԫǿ��������ϣ�c(OH��) �� c(H��)
�� pH��4��Ũ�Ⱦ�Ϊ0.1 mol��L��1��CH3COOH��CH3COONa�����Һ�У�c(CH3COO��)��c(OH��) �� c(CH3COOH)��c(H+)
A���٢ڢ� B���٢ۢ� C���ۢݢ� D���ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й�ԭ�ӽṹ��Ԫ�������ɡ�Ԫ�����ڱ���������ȷ����( )
A����IA��Ԫ��蘆�����ͬλ��137Cs��133Cs��4������
B��Ԫ�����ڱ���ʮ�˸����У���ʮ������
C���� ��A��Ԫ�ش��ϵ��£����⻯����ȶ�������ǿ
D��ͬ����Ԫ�ش��ϵ��£����ʵ��۵�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�֡���ʳ�����Ϳ�͡��ʺ��������ʱʹ�á����ڲ��������������ġ����Ѽӹ��õ���հ�װʳƷ��������Ƿֱ��װ��������ѧ���ʣ�ʹ��ʱ����Ԥ����������ߣ�ʹ�����ֻ�ѧ���ʷ�Ӧ����ʱ��ɶ�ʳ����м��ȣ���������ѧ��������ʵ�ѡ���ǡ�
A��Ũ������ˮ B����ʯ����ˮ C����ʯ���� D���Ȼ�����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(N A��ʾ�����ӵ³���)
A��1molCu��������Ӧ����Cu2S��ת�Ƶĵ�����Ϊ2 N A
B��R2+��������ΪA��������ΪN����ngR�ü�̬���������к�������
 N A
N A
C���Ȼ�������Fe(OH)3 ��������ӷ���ʽ��Fe3��+3H2O =Fe(OH)3��+3H��
D���ڼ�����Ƭ��������������Һ��һ������������飺Na+��Ba2+��Cl����NO3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com