

���� ��1��Fe��OH��2��������ˮ��Ӧ����Fe��OH��3���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��2���̷���FeSO4•7H2O���ױ��ʣ�Fe2+������ΪFe3+�������̷��Ƿ���ʵ��Լ���KSCN��Һ�������̷��Ƿ���ȫ���ʼ�������Ʒ���Ƿ����Fe2+��������Fe2+�Ļ�ԭ�Խ��м���������軯����Һ���飬ʵ�鷽��Ϊȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ�
��3����FeCO3•nH2O����������ΪFeOOH������������ԭ��Ӧ�����غ��ԭ���غ���ƽ��д��
����������Ԫ���غ��������ϵ���㣮
��� �⣺��1��Fe��OH��2������ˮ��Ӧ����Fe��OH��3����Ӧ�Ļ�ѧ����ʽΪ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��2���̷���FeSO4•7H2O���ױ��ʣ�Fe2+������ΪFe3+�������̷��Ƿ���ʵ��Լ���KSCN��Һ�������̷��Ƿ���ȫ���ʼ�������Ʒ���Ƿ����Fe2+��������Fe2+�Ļ�ԭ�Խ��м���������軯����Һ���飬ʵ�鷽��Ϊȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ���
�ʴ�Ϊ��KSCN��Һ��ȡ��Ʒ����ˮ���μ����Ը��������Һ������Һ��ɫ�����ʾ��Ʒû����ȫ���ʣ���μ����軯����Һ��������ɫ����������Ʒû����ȫ���ʣ���
��3���ٸ��������Ҫ��Ϊ����ȥ����ˮ�ͽᾧˮ�������л�������FeCO3•nH2O����������ΪFeOOH������������ԭ��Ӧ�ĵ����غ��ԭ���غ㣬д����ѧ����ʽΪ��4FeCO3•nH2O+O2=4FeOOH+4CO2��+��4n-2��H2O��
�ʴ�Ϊ��4FeCO3•nH2O+O2=4FeOOH+4CO2��+��4n-2��H2O��
������Ʒ��FeCO3�����ʵ���Ϊxmol��FeOOH�����ʵ���Ϊymol����
116x+89y=12.49��x+y=$\frac{6.16g}{56g/mol}$=0.11
���y=0.01mol����Ʒ������FeOOH������Ϊ0.89g��
�ʴ�Ϊ��0.89g��
���� ���⿼�����Ʊ���������ƣ���Ŀ�Ѷ��еȣ��漰��ѧ����ʽ��д�����Ӽ��顢��ѧ�����֪ʶ����ȷʵ��Ŀ�ġ�ʵ��ԭ��Ϊ���ؼ�������������ѧ���ķ�����������������ѧʵ�顢��ѧ����������


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.56L | B�� | 1.12L | C�� | 2.24L | D�� | 4.48L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CO��g��+2H2��g���TCH3OH��l�� | |
| B�� | 2Cu��s��+O2��g���T2CuO��s�� | |
| C�� | NH3��g��+HCl��g���TNH4Cl��s�� | |
| D�� | CaCO3��s��+2HCl��aq���TCaCl2��aq��+CO2��g��+H2O��l�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϵͳ���������л��CH3��2CHCH��CH2CH3����CH2CH2CH3��������Ϊ��2-��-3-�һ����� | |
| B�� | Ӳ֬��������������������Ϊͬϵ�� | |
| C�� | �������ױ��ķ���ʽΪC7H5N3O6 | |
| D�� | ��������ά�صĻ�ѧʽΪ��C6H10O5��n�������߲���ͬ���칹�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 2��4-����-3��3-���һ����� | |
| B�� | 3��3��5-��-4��4-���һ����� | |
| C�� | 3��3��-����-3-�һ�-3-��������� | |
| D�� | 2��4��4-����-3��3-���һ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ��ص�������п | B�� | ͭ�缫������ԭ��Ӧ | ||
| C�� | ԭ�����Cu2+��п���ƶ� | D�� | ͭ��������ų� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
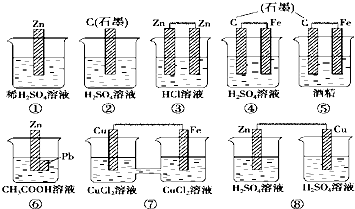
| A�� | �٢ۢ� | B�� | �ܢ� | C�� | �ܢޢ� | D�� | �٢ߢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | V=896 | B�� | ��ҺA�е�������ΪFe2+��Fe3+��H+ | ||
| C�� | ��Ʒ��CuO������Ϊ4.0g | D�� | ��Ʒ��FeԪ�ص�����Ϊ2.24g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ������� | B�� | ��Ӧ�¶� | C�� | Ũ�� | D�� | ѹǿ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com