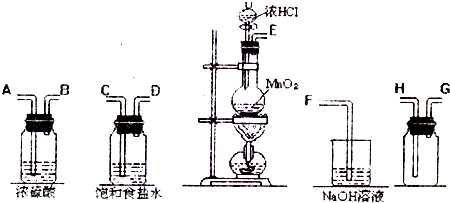
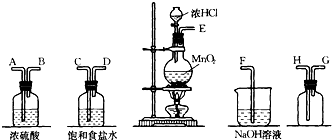
ŹµŃéŹŅÖŠÓƶžŃõ»ÆĆĢøśÅØŃĪĖį·“Ó¦ÖʱøøÉŌļ“æ¾»µÄĀČĘų£®ŹµŃéĖłÓĆŅĒĘ÷ČēĻĀ£ŗ
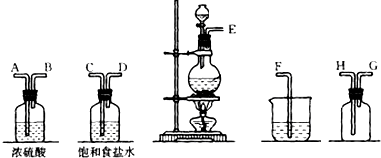
£Ø1£©Š“³ö·¢Éś×°ÖĆÖŠ½ųŠŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
MnO
2+4HCl£ØÅØ£©
MnCl
2+Cl
2ӟ+2H
2O
MnO
2+4HCl£ØÅØ£©
MnCl
2+Cl
2ӟ+2H
2O
£»
£Ø2£©ÉĻŹöŅĒĘ÷µÄĮ¬½ÓĖ³ŠņŹĒ£ØĢī½ÓæŚ×ÖÄø£©£ŗE½Ó
C
C
£¬
D
D
½Ó
A
A
£¬
B
B
½Ó
H
H
£¬
G
G
½Ó
F
F
£®
£Ø3£©ŹµŃéĒ°£¬Ó¦øĆ½ųŠŠµÄŅ»Ļī²Ł×÷ŹĒ
¼ģ²é×°ÖĆĘųĆÜŠŌ
¼ģ²é×°ÖĆĘųĆÜŠŌ
£®
£Ø4£©±„ŗĶŹ³ŃĪĖ®µÄ×÷ÓĆŹĒ
ĪüŹÕCl2ÖŠHCl
ĪüŹÕCl2ÖŠHCl
£®
£Ø5£©ČōŹµŃéÖŠµĆµ½±źæöĻĀ11.2LĀČĘų£¬±»Ńõ»ÆµÄHClµÄĪļÖŹµÄĮæĪŖ
1mol
1mol
£¬“ĖŹ±×ŖŅʵĵē×ÓŹżŹĒ
NA
NA
£®
£Ø6£©ĻĀĮŠŹŌ¼ĮÖŠ£¬æÉŅŌÓĆĄ“ĪüŹÕ²ŠÓąĀČĘųµÄŹĒ
BC
BC
£®
A£®±„ŗĶŹ³ŃĪĖ®£» B£®ĒāŃõ»ÆÄĘČÜŅŗ£» C£®Ģ¼ĖįÄĘČÜŅŗ£» D£®ÅØĮņĖį
£Ø7£©¹¤ŅµÉĻÖĘČ”ĀČĘųµÄĄė×Ó·½³ĢŹ½ĪŖ
2Cl
-+2H
2O
Cl
2ӟ+H
2ӟ+2OH
-2Cl
-+2H
2O
Cl
2ӟ+H
2ӟ+2OH
-£®




 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø