
 ��
��
 ��д����������Ӧ������Լ�������Һ�塢�廯�����������塢����
��д����������Ӧ������Լ�������Һ�塢�廯�����������塢����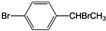 ����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��
����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ�� ��
������ ��1�����屽��ϩ���ϩ��һ�������·����Ӿ۷�Ӧ���ɸ߷��ӻ����
��2���ұ����� ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯����������֧����Hԭ�ӱ�ȡ����Ҫ����������
ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯����������֧����Hԭ�ӱ�ȡ����Ҫ����������
��3�� ����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ�����ݴ��ж�A�ṹ��ʽ��A����������ȥ��Ӧ���ɶ��屽��ϩ��
����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ�����ݴ��ж�A�ṹ��ʽ��A����������ȥ��Ӧ���ɶ��屽��ϩ��
��4��B��2��3-����-1-��ϩ��Ϊͬ���칹�壬������̼ԭ�Ӵ���ͬһƽ�棬��B���ĸ�������̼̼˫������̼ԭ�ӣ���ṹ��ʽΪ��CH3��2C=C��CH3��2��
2��3-����-1-��ϩ��HBr�����ӳɷ�Ӧ���ɣ�CH3��2CBrCH��CH3��2����CH3��2CBrCH��CH3��2������ȥ��Ӧ����
��CH3��2C=C��CH3��2��
��� �⣺��1�����屽��ϩ���ϩ��һ�������·����Ӿ۷�Ӧ���ɸ߷��ӻ������ṹ��ʽ ��
�� ��
��
�ʴ�Ϊ��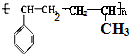 ��
��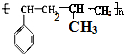 ��
��
��2���ұ����� ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯������������ҪҺ������Ӧ�֧����Hԭ�ӱ�ȡ����Ҫ������������Ҫ������Ӧ��ʴ�Ϊ��Һ�塢�廯�����������塢���գ�
ʱ��������Hԭ�ӱ�ȡ����Ҫ�廯������������ҪҺ������Ӧ�֧����Hԭ�ӱ�ȡ����Ҫ������������Ҫ������Ӧ��ʴ�Ϊ��Һ�塢�廯�����������塢���գ�
��3�� ����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ������A�ṹ��ʽΪ
����������������Һ���ȵõ�A��A��������������FeCl3��Һ����ɫ��˵��A��û�з��ǻ�����ֻ��֧����Brԭ�ӱ�-OHȡ������A�ṹ��ʽΪ ��A��Ũ���ᡢ���������·�����ȥ��Ӧ���ɶ��屽��ϩ��
��A��Ũ���ᡢ���������·�����ȥ��Ӧ���ɶ��屽��ϩ��
�ʴ�Ϊ�� ��Ũ���ᡢ���ȣ�
��Ũ���ᡢ���ȣ�
��4��B��2��3-����-1-��ϩ��Ϊͬ���칹�壬������̼ԭ�Ӵ���ͬһƽ�棬��B���ĸ�������̼̼˫������̼ԭ�ӣ���ṹ��ʽΪ��CH3��2C=C��CH3��2��
2��3-����-1-��ϩ��HBr�����ӳɷ�Ӧ���ɣ�CH3��2CBrCH��CH3��2����CH3��2CBrCH��CH3��2������ȥ��Ӧ����
��CH3��2C=C��CH3��2��������ͼΪCH2=C��CH3��C��CH3��2$��_{һ��������}^{HBr}$��CH3��2CBrCH��CH3��2$��_{��}^{NaOH�Ĵ���Һ}$��CH3��2C=C��CH3��2��
�ʴ�Ϊ����CH3��2C=C��CH3��2��CH2=C��CH3��C��CH3��2$��_{һ��������}^{HBr}$��CH3��2CBrCH��CH3��2$��_{��}^{NaOH�Ĵ���Һ}$��CH3��2C=C��CH3��2��
���� ����Ϊ2015��߿��⣬�����л���ѧ�ϳɣ�Ϊ��Ƶ���㣬���ؿ���ѧ��֪ʶ�ۺ�Ӧ����������Ҫѧ���Գ����л�������ż������ʡ�������Ӧ���ͼ������������ղ�������ã��ѵ��Ǻϳ�·����Ƶģ�4���⣬��Ŀ�ѶȽϴ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ����� | Ԥ������ | ���� |
| ����һ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �ڳ����Ļ������У����̬�ӵ����ߵ�˳���ǣ�C��N��O=S��Cl | |
| B�� | ԭ��������С�����ǣ�C��N��O��S��Cl | |
| C�� | ����Ԫ�����ڱ������������������Ӵ���С���ǣ�Cl��O��S��N��C | |
| D�� | ԭ�ӵ������������ɶ����ٵ�˳���ǣ�Cl��O=S��N��C |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | þ�� | B�� | �⻯�� | C�� | �� | D�� | ���Ȼ�̼ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ԫ�ر��ĵ��ʿ�����ұ������ | B�� | ���붡�γɵķ������зǼ��Է��� | ||
| C�� | �����Ӱ뾶�������ң��� | D�� | �������γɵĻ�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʯ��ׯ������ѧ�߶��Ͻο�һ��ѧ���������棩 ���ͣ������
�����ʵ�������ϩ����飬����������֮��Ϊ_____________��̼ԭ�Ӹ���֮��Ϊ_____________����ԭ�Ӹ���֮��Ϊ______________�������ȼ�գ�����O2�������Ϊ_________��0.1 molij������������������ȫȼ�գ�����CO2��ˮ��0.6 mol��������ķ���ʽΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʯ��ׯ������ѧ�߶��Ͻο�һ��ѧ���������棩 ���ͣ�ѡ����
�������ʲ���������ϩ�ӳɲ�����ǣ� ��
A��CH3CH3 B��CH3CHCl2 C��CH3CH2OH D��CH3CH2Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʯ��ׯ������ѧ�߶��Ͻο�һ��ѧ���������棩 ���ͣ�ѡ����
������ʵ����˵�������ᣨHNO2����������ʵ��ǣ� ��
A��HNO2����NaCl��Ӧ
B��NaNO2��Һ��pH����7
C��������0.l mol•L��1 HNO2��Һ��pHΪ2.145
D����ͬ�¶��£�����ͬŨ�ȵ�HCl��Һ��HNO2��Һ���������飬HNO2��Һ���ݽϰ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A��������ȼ����Ϊ��H=-285.5kJ•mol��1������ˮ���Ȼ�ѧ����ʽΪ2H2O ��l�� 2H2��g��+O2��g�� ��H=+285.5k•Jmol��1
2H2��g��+O2��g�� ��H=+285.5k•Jmol��1
B����ӦSO2��g��+2H2S��g��=3S��s��+2H2O��l���ڳ��������Է����У���÷�Ӧ�ġ�H��0
C��500�桢30MPa�£���0.5molN2��1.5molH2�����ܱյ������г�ַ�Ӧ����NH3��g��������19.3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g�� 2NH3��g����H=-38.6k•Jmol��1
2NH3��g����H=-38.6k•Jmol��1
D����C��ʯī��s��=C�����ʯ��s����H=+1.90k•Jmol��1��֪�����ʯ��ʯī�ȶ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com