
| ””ĪļÖŹ | Ēā¼üX-H”Y”” | ¼üÄÜkJ£®mol-1 |
| ””£ØHF£©n | ””D-H”F | ””28.1 |
| ””±ł | ””O-H”O | ””18.8 |
| ””£ØNH3£©n | ””N-H”N | ””5.4 |

·ÖĪö £Ø1£©Ķ¬Ņ»ÖÜĘŚŌŖĖŲÖŠ£¬ŌŖĖŲµÄµŚŅ»µēĄėÄÜĖę×ÅŌ×ÓŠņŹżµÄŌö“ó¶ų³ŹŌö“óĒ÷ŹĘ£¬µ«µŚIIA×唢µŚVA×åŌŖĖŲµŚŅ»µēĄėÄÜ“óÓŚĶ¬ÖÜĘŚĻąĮŚŌŖĖŲµÄ£»
£Ø2£©Ä³ŌŖĖŲĪ»ÓŚµŚĖÄÖÜĘŚ¢ų×壬Ę仳Ģ¬Ō×ÓµÄĪ“³É¶Ōµē×ÓŹżÓė»łĢ¬Ģ¼Ō×ÓµÄĪ“³É¶Ōµē×ÓŹżĻąĶ¬£¬Ģ¼Ō×ӵĵē×ÓÅŲ¼ĪŖ1s22s22p2£¬Ī“³É¶Ōµē×ÓŹżĪŖ2£¬ŌņøĆŌŖĖŲĪŖNi£»
£Ø3£©ŅŅĻ©ĶŖ·Ö×ÓÖŠĢ¼Ō×Ó¾łĆ»ÓŠ¹Ā¶Ōµē×Ó£¬CH2ÖŠCŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬¶ųC=OÖŠĢ¼Ō×ÓŠĪ³É2øö¦Ņ¼ü£¬ŌӻƹģµĄŹżÄæ·Ö±šĪŖ3”¢2£»£ØC2H5O£©3P=O·Ö×Óŗ¬ÓŠ25øö¦Ņ¼ü£»
£Ø4£©Ę½¾łĆæøö·Ö×Óŗ¬Ēā¼üŹż£ŗ±łÖŠ2øö£¬£ØHF£©nŗĶ£ØNH3£©nÖ»ÓŠ1øö£¬½įŗĻĘų»ÆŅŖæĖ·žµÄĒā¼üµÄ×ܼüÄÜ·ÖĪö£»
£Ø5£©øł¾Ż¾łĢƷؼĘĖć¾§°ūÖŠSi”¢CŌ×ÓŹżÄ棬ĆæøöSiŌ×ÓÖÜĪ§ÓŠ4øöĢ¼Ō×Ó£¬Ō×ÓÅäĪ»ŹżÓėŌ×ÓŹżÄæ³É·“±Č£¬æÉŅŌ¼ĘĖćĢ¼Ō×ÓÖÜĪ§ÓėĘä¾ąĄė×ī½üµÄ¹čŌ×ÓŹżÄ棻
ŅŌ¶„µćCŌ×ÓŃŠ¾æ£¬ÓėÖ®¾ąĄė×ī½üµÄCŌ×ÓĪ»ÓŚĆęŠÄÉĻ£¬Ćæøö¶„µćŌ×ÓĪŖ8øö¾§°ū¹²ÓĆ£¬ĆæøöĆęĪŖ2øö¾§°ū¹²ÓĆ£»
×÷¹ż1ŗÅSiŌ×ÓµÄĢå¶Ō½ĒĻß”¢2ŗÅĢ¼Ō×ÓµÄĢå¶Ō½ĒĻߣ¬Ļą½»ÓŚOµć£¬Óė¶„µćĢ¼Ō×ÓŠĪ³ÉČēĶ¼ĖłŹ¾£ŗ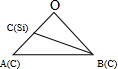 £¬ĘäÖŠBĪŖ2ŗÅĢ¼Ō×Ó£¬CĪŖ1ŗÅSiŌ×Ó£¬1ŗÅSiŌ×ÓÓėÖÜĪ§µÄ4øöCŌ×ÓŠĪ³ÉÕżĖÄĆęĢ壬1ŗÅSiŌ×ÓÓė¶„µćĢ¼Ō×ÓĮ¬Ļß“¦ÓŚ¾§°ūĢå¶Ō½ĒĻßÉĻ£¬ĒŅ¾ąĄėĪŖĢå¶Ō½ĒĻß³¤¶ČµÄ$\frac{1}{4}$£¬Ģå¶Ō½ĒĻß³¤¶ČĪŖ$\sqrt{3}$a pm£¬ŌņOA=OB=$\frac{\sqrt{3}}{2}$a pm£¬¹ŹOC=$\frac{\sqrt{3}}{4}$a pm£¬ĄūÓĆÓąĻŅ¶ØĄķ¼ĘĖćcos”ĻAOBµÄÖµ£¬ŌŁĄūÓĆÓąĻŅ¶ØĄķ¼ĘĖćBCµÄ³¤¶Č£¬¼“¾§°ūĶ¼2ÖŠ1ŗŹčŌ×ÓŗĶ2ŗÅĢ¼Ō×ÓÖ®¼äµÄ¾ąĄė£»
£¬ĘäÖŠBĪŖ2ŗÅĢ¼Ō×Ó£¬CĪŖ1ŗÅSiŌ×Ó£¬1ŗÅSiŌ×ÓÓėÖÜĪ§µÄ4øöCŌ×ÓŠĪ³ÉÕżĖÄĆęĢ壬1ŗÅSiŌ×ÓÓė¶„µćĢ¼Ō×ÓĮ¬Ļß“¦ÓŚ¾§°ūĢå¶Ō½ĒĻßÉĻ£¬ĒŅ¾ąĄėĪŖĢå¶Ō½ĒĻß³¤¶ČµÄ$\frac{1}{4}$£¬Ģå¶Ō½ĒĻß³¤¶ČĪŖ$\sqrt{3}$a pm£¬ŌņOA=OB=$\frac{\sqrt{3}}{2}$a pm£¬¹ŹOC=$\frac{\sqrt{3}}{4}$a pm£¬ĄūÓĆÓąĻŅ¶ØĄķ¼ĘĖćcos”ĻAOBµÄÖµ£¬ŌŁĄūÓĆÓąĻŅ¶ØĄķ¼ĘĖćBCµÄ³¤¶Č£¬¼“¾§°ūĶ¼2ÖŠ1ŗŹčŌ×ÓŗĶ2ŗÅĢ¼Ō×ÓÖ®¼äµÄ¾ąĄė£»
½įŗĻ¾§°ūÖŠŌ×ÓŹżÄ棬±ķŹ¾³ö¾§°ūÖŹĮ棬ŌŁøł¾Ż¦Ń=$\frac{m}{V}$¼ĘĖć¾§ĢåĆÜ¶Č£®
½ā“š ½ā£ŗ£Ø1£©Ķ¬Ņ»ÖÜĘŚŌŖĖŲÖŠ£¬ŌŖĖŲµÄµŚŅ»µēĄėÄÜĖę×ÅŌ×ÓŠņŹżµÄŌö“ó¶ų³ŹŌö“óĒ÷ŹĘ£¬°ė³äĀśµÄNŌ×ÓŗĶČ«³äĀśµÄBeŌ×ÓµŚŅ»µēĄėÄÜŅŖ±ČĶ¬ÖÜĘŚĻąĮŚŌŖĖŲµÄøߣ¬¹ŹµŚŅ»µēĄėÄܽéÓŚB”¢NÖ®¼äµÄµŚ¶žÖÜĘŚŌŖĖŲÓŠBe”¢C”¢OČżÖÖŌŖĖŲ£¬
¹Ź“š°øĪŖ£ŗ3£»
£Ø2£©Ä³ŌŖĖŲĪ»ÓŚµŚĖÄÖÜĘŚ¢ų×壬Ę仳Ģ¬Ō×ÓµÄĪ“³É¶Ōµē×ÓŹżÓė»łĢ¬Ģ¼Ō×ÓµÄĪ“³É¶Ōµē×ÓŹżĻąĶ¬£¬CŌ×ӵĵē×ÓÅŲ¼ĪŖ1s22s22p2£¬Ī“³É¶Ōµē×ÓŹżĪŖ2£¬ŌņøĆŌŖĖŲĪŖNi£¬Ę仳Ģ¬Ō×ӵļŪ²ćµē×ÓÅŲ¼Ź½ĪŖ3d84s2£¬
¹Ź“š°øĪŖ£ŗ3d84s2£»
£Ø3£©ŅŅĻ©ĶŖ·Ö×ÓÖŠĢ¼Ō×Ó¾łĆ»ÓŠ¹Ā¶Ōµē×Ó£¬CH2ÖŠCŌ×ÓŠĪ³É3øö¦Ņ¼ü£¬¶ųC=OÖŠĢ¼Ō×ÓŠĪ³É2øö¦Ņ¼ü£¬ŌӻƹģµĄŹżÄæ·Ö±šĪŖ3”¢2£¬ĖłŅŌĢ¼Ō×ÓµÄŌӻƹģµĄĄąŠĶÓŠsp2ŗĶsp£¬£ØC2H5O£©3P=O·Ö×Óŗ¬ÓŠ25øö¦Ņ¼ü£¬1mol£ØC2H5O£©3P=O·Ö×ÓÖŠŗ¬ÓŠµÄ¦Ņ¼üµÄŹżÄæĪŖ25NA£¬
¹Ź“š°øĪŖ£ŗsp2ŗĶsp£»25NA£»
£Ø4£©µ„øöĒā¼üµÄ¼üÄÜŹĒ£ØHF£©n£¾±ł£¾£ØNH3£©n£¬¶ųĘ½¾łĆæøö·Ö×Óŗ¬Ēā¼üŹż£ŗ±łÖŠ2øö£¬£ØHF£©nŗĶ£ØNH3£©nÖ»ÓŠ1øö£¬Ęų»ÆŅŖæĖ·žµÄĒā¼üµÄ×ܼüÄÜŹĒ±ł£¾£ØHF£©n£¾£ØNH3£©n£¬¹ŹH2O”¢HF”¢NH3·ŠµćŅĄ“Ī½µµĶ£¬
¹Ź“š°øĪŖ£ŗµ„øöĒā¼üµÄ¼üÄÜŹĒ£ØHF£©n£¾±ł£¾£ØNH3£©n£¬¶ųĘ½¾łĆæøö·Ö×Óŗ¬Ēā¼üŹż£ŗ±łÖŠ2øö£¬£ØHF£©nŗĶ£ØNH3£©nÖ»ÓŠ1øö£¬Ęų»ÆŅŖæĖ·žµÄĒā¼üµÄ×ܼüÄÜŹĒ±ł£¾£ØHF£©n£¾£ØNH3£©n£»
£Ø5£©¾§°ūÖŠSiŌ×ÓŹżÄæĪŖ4”¢CŌ×ÓŹżÄæĪŖ8”Į$\frac{1}{8}$+6”Į$\frac{1}{2}$=4£¬ĆæøöSiŌ×ÓÖÜĪ§ÓŠ4øöĢ¼Ō×Ó£¬Ō×ÓÅäĪ»ŹżÓėŌ×ÓŹżÄæ³É·“±Č£¬ŌņĢ¼Ō×ÓÅäĪ»ŹżŅ²ŹĒ4£¬¼“Ģ¼Ō×ÓÖÜĪ§ÓėĘä¾ąĄė×ī½üµÄ¹čŌ×ÓŹżÄæĪŖ4£»
ŅŌ¶„µćCŌ×ÓŃŠ¾æ£¬ÓėÖ®¾ąĄė×ī½üµÄCŌ×ÓĪ»ÓŚĆęŠÄÉĻ£¬Ćæøö¶„µćŌ×ÓĪŖ8øö¾§°ū¹²ÓĆ£¬ĆæøöĆęĪŖ2øö¾§°ū¹²ÓĆ£¬ÓėĢ¼Ō×ÓµČ¾ąĄė×ī½üµÄĢ¼Ō×ÓÓŠ$\frac{8”Į3}{2}$=12øö£»
×÷¹ż1ŗÅSiŌ×ÓµÄĢå¶Ō½ĒĻß”¢2ŗÅĢ¼Ō×ÓµÄĢå¶Ō½ĒĻߣ¬Ļą½»ÓŚOµć£¬Óė¶„µćĢ¼Ō×ÓŠĪ³ÉČēĶ¼ĖłŹ¾£ŗ £¬ĘäÖŠBĪŖ2ŗÅĢ¼Ō×Ó£¬CĪŖ1ŗÅSiŌ×Ó£¬1ŗÅSiŌ×ÓÓėÖÜĪ§µÄ4øöCŌ×ÓŠĪ³ÉÕżĖÄĆęĢ壬1ŗÅSiŌ×ÓÓė¶„µćĢ¼Ō×ÓĮ¬Ļß“¦ÓŚ¾§°ūĢå¶Ō½ĒĻßÉĻ£¬ĒŅ¾ąĄėĪŖĢå¶Ō½ĒĻß³¤¶ČµÄ$\frac{1}{4}$£¬Ģå¶Ō½ĒĻß³¤¶ČĪŖ$\sqrt{3}$a pm£¬ŌņOA=OB=$\frac{\sqrt{3}}{2}$a pm£¬¹ŹOC=$\frac{\sqrt{3}}{4}$a pm£¬Ōņ£ŗ
£¬ĘäÖŠBĪŖ2ŗÅĢ¼Ō×Ó£¬CĪŖ1ŗÅSiŌ×Ó£¬1ŗÅSiŌ×ÓÓėÖÜĪ§µÄ4øöCŌ×ÓŠĪ³ÉÕżĖÄĆęĢ壬1ŗÅSiŌ×ÓÓė¶„µćĢ¼Ō×ÓĮ¬Ļß“¦ÓŚ¾§°ūĢå¶Ō½ĒĻßÉĻ£¬ĒŅ¾ąĄėĪŖĢå¶Ō½ĒĻß³¤¶ČµÄ$\frac{1}{4}$£¬Ģå¶Ō½ĒĻß³¤¶ČĪŖ$\sqrt{3}$a pm£¬ŌņOA=OB=$\frac{\sqrt{3}}{2}$a pm£¬¹ŹOC=$\frac{\sqrt{3}}{4}$a pm£¬Ōņ£ŗ
£Ø$\frac{\sqrt{3}}{2}$a£©2+£Ø$\frac{\sqrt{3}}{2}$a£©2-2”Į$\frac{\sqrt{3}}{2}$a”Į$\frac{\sqrt{3}}{2}$a”Įcos”ĻAOB=a2£¬
½āµĆcos”ĻAOB=$\frac{1}{3}$
¹Ź£Ø$\frac{\sqrt{3}}{4}$a£©2+£Ø$\frac{\sqrt{3}}{2}$a£©2-2”Į$\frac{\sqrt{3}}{4}$a”Į$\frac{\sqrt{3}}{2}$a”Į$\frac{1}{3}$=BC2£¬
½āµĆBC=$\frac{\sqrt{11}a}{4}$
¾§°ūÖŹĮæĪŖ4”Į$\frac{28+12}{{N}_{A}}$g£¬Ōņ¾§ĢåĆܶČĪŖ4”Į$\frac{28+12}{{N}_{A}}$g”Ā£Øa”Į10-10 cm£©3=$\frac{1.6”Į1{0}^{32}}{{a}^{3}”Į{N}_{A}}$g/cm3£¬
¹Ź“š°øĪŖ£ŗ4£»12£»$\frac{\sqrt{11}a}{4}$£»$\frac{1.6”Į1{0}^{32}}{{a}^{3}”Į{N}_{A}}$£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹ÓėŠŌÖŹµÄ漲飬Éę¼°ŗĖĶāµē×ÓÅŲ¼”¢µēĄėÄÜ”¢ŌӻƹģµĄ”¢»Æѧ¼ü”¢Ēā¼ü”¢¾§°ū¼ĘĖćµČ£¬£Ø4£©£Ø5£©ĪŖŅדķµć”¢ÄŃµć£¬²ąÖŲæ¼²éѧɜ·ÖĪö¼ĘĖćÄÜĮ¦£¬ÄŃ¶Č½Ļ“ó£®


 æŖŠÄĶÜæŚĖćĢāæØĻµĮŠ“š°ø
æŖŠÄĶÜæŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | c£ØCO32-£© | B£® | c£ØMg2+£© | C£® | c£ØH+£© | D£® | Ksp£ØMgCO3£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
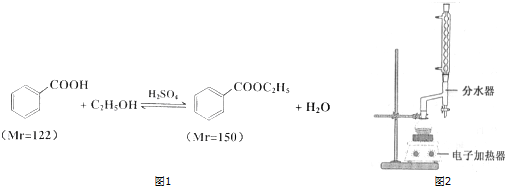
| ŃÕÉ«”¢×“Ģ¬ | ·Šµć£Ø”ę£© | ĆÜ¶Č£Øg•cm-3£© | |
| ±½¼×Ėį* | ĪŽÉ«”¢Ę¬×“¾§Ģå | 249 | 1.2659 |
| ±½¼×ĖįŅŅõ„ | ĪŽÉ«³ĪĒåŅŗĢå | 212.6 | 1.05 |
| ŅŅ“¼ | ĪŽÉ«³ĪĒåŅŗĢå | 78.3 | 0.7893 |
| »·¼ŗĶé | ĪŽÉ«³ĪĒåŅŗĢå | 80.8 | 0.7318 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
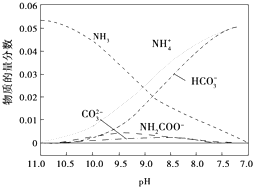
| A£® | ŌŚpH=9.0Ź±£¬c£ØNH4+£©£¾c£ØHCO3-£©£¾c£ØNH2COO-£©£¾c£ØCO32-£© | |
| B£® | ²»Ķ¬pHµÄČÜŅŗÖŠ“ęŌŚ¹ŲĻµ£ŗc£ØNH4+£©+c£ØH+£©ØT2c£ØCO32-£©+c£ØHCO3-£©+c£ØNH2COO-£©+c£ØOH-£© | |
| C£® | ŌŚČÜŅŗpH²»¶Ļ½µµĶµÄ¹ż³ĢÖŠ£¬ÓŠŗ¬NH2COO-µÄÖŠ¼ä²śĪļÉś³É | |
| D£® | Ėę×ÅCO2µÄĶØČė£¬$\frac{c£ØO{H}^{-}£©}{c£ØN{H}_{3}•{H}_{2}O£©}$²»¶ĻŌö“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ÓƶčŠŌµē¼«µē½āŗ¬·ÓĢŖµÄ±„ŗĶŹ³ŃĪĖ®£¬Ńō¼«ø½½üČÜŅŗĻȱäŗģ | |
| B£® | ĶµÄµē½ā¾«Į¶¹ż³ĢÖŠ£¬ÓŠ0.2 mol µē×Ó·¢Éś×ŖŅĘŹ±£¬Ńō¼«Ņ»¶ØÓŠ6.4 gĶČܽā | |
| C£® | ½«µŲĻĀøÖ¹ÜÓėÖ±Į÷µēŌ“µÄÕż¼«ĻąĮ¬£¬³ĘĪŖĶā¼ÓµēŌ“µÄŅõ¼«±£»¤·Ø | |
| D£® | ŌŚÖŠŠŌ»·¾³ÖŠ£¬ĆæÉś³É35.6 gĢśŠā£ØFe2O3•H2O£©£¬ĻūŗĵÄŃõĘųĪŖ6.72 L£Ø±ź×¼×“æö£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
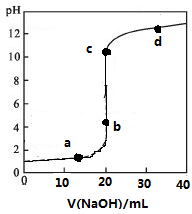 ³£ĪĀĻĀ£¬Ļņ20mL0.1mol/LijĖįHRČÜŅŗÖŠµĪ¼Ó0.1mol/LĒāŃõ»ÆÄĘČÜŅŗ£¬ČÜŅŗµÄPHÓėĒāŃõ»ÆÄĘČÜŅŗĢå»żVµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©
³£ĪĀĻĀ£¬Ļņ20mL0.1mol/LijĖįHRČÜŅŗÖŠµĪ¼Ó0.1mol/LĒāŃõ»ÆÄĘČÜŅŗ£¬ČÜŅŗµÄPHÓėĒāŃõ»ÆÄĘČÜŅŗĢå»żVµÄ¹ŲĻµČēĶ¼ĖłŹ¾£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ£Ø””””£©| A£® | æÉŃ”·ÓĢŖ»ņ¼×»ł³Č×÷ÖøŹ¾¼Į | |
| B£® | µĪ¶ØĒ°HRČÜŅŗÖŠ“ęŌŚ“óĮæHR·Ö×Ó | |
| C£® | V=20 mLŹ±£¬ČÜŅŗÖŠĖ®µēĄėµÄ£ŗc£ØH+£©”Įc£ØOH-£©=1”Į10-14mol2/L2 | |
| D£® | cµćŹ±ČÜŅŗÖŠĄė×ÓÅØ¶Č“óŠ”¹ŲĻµÓŠc£ØNa+£©£¾c£ØR-£©£¾c£ØOH-£©£¾c£ØH+£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
 øßĆĢĖį¼ŲŹĒŅ»ÖÖÖŲŅŖµÄ»ÆѧŹŌ¼Į£¬ĘäČÜŅŗ²»ŗÜĪČ¶Ø£¬ŌŚĖįŠŌĢõ¼žĻĀ»į·Ö½āÉś³É¶žŃõ»ÆĆĢŗĶŃõĘų£¬ŌŚÖŠŠŌ»ņČõ¼īŠŌČÜŅŗÖŠ·Ö½āĖŁ¶ČŗÜĀż£¬¼ū¹ā·Ö½āĖŁ¶Č¼Óæģ£®
øßĆĢĖį¼ŲŹĒŅ»ÖÖÖŲŅŖµÄ»ÆѧŹŌ¼Į£¬ĘäČÜŅŗ²»ŗÜĪČ¶Ø£¬ŌŚĖįŠŌĢõ¼žĻĀ»į·Ö½āÉś³É¶žŃõ»ÆĆĢŗĶŃõĘų£¬ŌŚÖŠŠŌ»ņČõ¼īŠŌČÜŅŗÖŠ·Ö½āĖŁ¶ČŗÜĀż£¬¼ū¹ā·Ö½āĖŁ¶Č¼Óæģ£®µĪČėøßĆĢĖį¼ŲČÜŅŗµÄ“ĪŠņ£ØĆæµĪČÜŅŗµÄĢå»żĻąĶ¬£© | øßĆĢĖį¼ŲČÜŅŗ×ĻÉ«ĶŹČ„µÄŹ±¼ä |
| ĻȵĪČėµŚ1µĪ | 1min |
| ĶŹÉ«ŗóŌŁµĪČėµŚ2µĪ | 15s |
| ĶŹÉ«ŗóŌŁµĪČėµŚ3µĪ | 3s |
| ĶŹÉ«ŗóŌŁµĪČėµŚ4µĪ | 1s |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | c£ØNa+£©=c£ØHA-£©+2c£ØA2-£©+c£ØOH-£© | |
| B£® | c£ØH2A£©+c£ØHA-£©+c£ØA2-£©=0.1 mol•L-1 | |
| C£® | ½«ÉĻŹöČÜŅŗĻ”ŹĶÖĮ0.01mol/L£¬c£ØH+£©•c£ØOH-£© ²»±ä | |
| D£® | c £ØA2-£©+c £ØOH-£©=c £ØH+£©+c £ØH2A£© |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com