
Ϊ��֤±�ص��������Ե����ǿ����ijС������ͼ��ʾװ�ý���ʵ��(�г���������ȥ���������Ѽ���)��
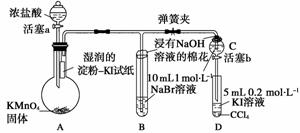
ʵ����̣�
��.���ɼУ�����a���μ�Ũ���ᡣ
��.��B��C�е���Һ����Ϊ��ɫʱ���н����ɼС�
��.��B����Һ�ɻ�ɫ��Ϊ����ɫʱ���رջ���a��
��.����
(1)A�в�������ɫ���壬�����ʽ��______________________________________��
(2)��֤������������ǿ�ڵ��ʵ��������__________________________________��
(3)B����Һ������Ӧ�����ӷ���ʽ��______________________________________��
(4)Ϊ��֤���������ǿ�ڵ⣬���̢��IJ�����������_________________________
________________________________________________________________________��
(5)���̢�ʵ���Ŀ����_______________________ ___________________________��
___________________________��
(6)�ȡ��塢�ⵥ�ʵ�������������ԭ��ͬ����Ԫ�ش��ϵ���____________________���õ�������������
�𰸡�(1) 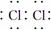
(2)ʪ��ĵ���KI��ֽ����
(3)Cl2��2Br��===Br2��2Cl��
(4)����b��������C����Һ����D�У��رջ���b��ȡ��D�������ú�CCl4����Һ��Ϊ�Ϻ�ɫ
(5)ȷ��C�Ļ�ɫ��Һ����Cl2���ų�Cl2�����û���ʵ��ĸ���
(6)ԭ�Ӱ뾶������
������A�еμ�Ũ���������Ӧ��2KMnO4��16HCl(Ũ)===2KCl��2MnCl2��5Cl2����8H2O�����ɻ���ɫ����Cl2����A��B��C�зֱ�����Ӧ��Cl2��2KI===2KCl��I2��Cl2��2NaBr===2NaCl��Br2��Cl2��2NaBr===2NaCl��Br2������B��C��������Br2��ʹ��Һ��Ϊ��ɫ������b��C�����ɵ�Br2��D�з�����Ӧ��Br2��2KI===2KBr��I2�����̢�ʵ�飬��B�л�ɫ��Һ����ͨ�����Cl2ʱ����Һ��Ϊ����ɫ���Դ�Ϊ���գ�˵��C�л�ɫ��Һ��Cl2���Ӷ��ų�Cl2�����û���ʵ��ĸ��š�


 53������ϵ�д�
53������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ʯ����һ���°����ʣ��ǡ�¹�ص���Լ���������Ƶ�46�ֻ�ѧƷ֮һ���仯ѧʽΪNa2Fe5Si8O22(OH)2����ʯ����ϡ������Һ����ʱ����ԭ����ֻ��NO������˵������ȷ���� (����)
A����ʯ����һ�ֹ����β���
B����ʯ���к���һ������ʯӢ����
C����ʯ�Ļ�ѧ��ɿɱ�ʾΪNa2O��3FeO��Fe2O3��8SiO2��H2O
D��1 mol��ʯ����ʹ1 mol HNO3����ԭ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����(����)
A����Ca(HCO3)2��Һ�м������NaOH��Һ��
Ca2����HCO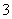 ��OH��===CaCO3����H2O
��OH��===CaCO3����H2O
B����ʯī�缫��ⱥ��ʳ��ˮ��
2H����2Cl��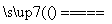 Cl2����H2��
Cl2����H2��
C��AlCl3��Һ������ˮ��Ӧ��
Al3����3OH��===Al(OH)3��
D������۵⻯����Һ�еμ�ϡ���ᣬ�ڿ����з���һ��ʱ��������4H����4I����O2===2I2��2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ�д��ڶ��ַ��Ӻ����ӣ������ڲ�ͬ�ķ�Ӧ�б��ֳ���ͬ�����ʡ����н�����ȷ���� (����)
A��������ɫ������Ƭ�̺���ɫ������ɫ��˵����Cl2����
B����Һ��dz����ɫ�����д̼�����ζ��˵����Cl2����
C���ȼ��������ữ���ټ���AgNO3��Һ�����ɰ�ɫ������˵����Cl������
D������NaOH��Һ����ˮ��dz����ɫ��ʧ��˵����HClO����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)��ˮ����Ĺ����в�����������ԭ��Ӧ (����)
(2)�����õ�����Һ����ӵ�ʳ���е�KIO3 (����)
(3)��CCl4��ȡ��ˮ�еĵ⣬�������ã�Һ��ֲ㣬�²����ɫ (����)
(4)�������������ü�����������ȥNH4Cl�л��е�I2 (����)
(5)��ʹʪ��ĵ���KI��ֽ����������һ����Cl2 (����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ж�������ȷ�Ļ����̡�������Ļ�������
(1)(2013���㽭���ۣ�8A)ʵ���ҴӺ�����ȡ���ʵ�ķ����ǣ�ȡ�������ա��ܽ�����ˡ���ȡ (����)
(2)�Ӻ�ˮ����ȡ���ʶ�����ͨ����ѧ��Ӧ����ʵ�� (����)
(2013���������ۣ�6B)
(3)����������Һ�����ڻ���������ɱ�� (����)
(2013���Ĵ����ۣ�1D)
(4)�ڡ����ͷ����Ԫ�صļ��顱ʵ���У�ժ�¼���δȼ���Ļ��ͷ���������ˮ�У��Ժ�ȡ������Һ���Թ��У��μ���������Һ��ϡ��������ж���Ԫ�صĴ���(����)
(2012���㽭���ۣ�8B)
(5)���ݽ�ǿ�������ȡ������Ĺ��ɣ��Ƴ�CO2ͨ��NaClO ��Һ��������HClO(����)
(2011���������ۣ�11D)
(6) ��ʪ���ֽ�����ڱ�����ˮ�����к�ֽ����ɫ (����)
��ʪ���ֽ�����ڱ�����ˮ�����к�ֽ����ɫ (����)
(2012���������ۣ�10D)
(7) �ٷ�̪��Һ����Ũ���ᡣ���������Ա仯 (����)
�ٷ�̪��Һ����Ũ���ᡣ���������Ա仯 (����)
(2012���������ۣ�10B)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ������װ���Ʊ�������Cl2�����ʡ�

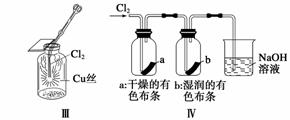
����˵����ȷ���� (����)
A����ͼ�У����MnO2������Ũ����Ϳ�ȫ��������
B����ͼ�У���Ͳ�з����˼ӳɷ�Ӧ
C����ͼ�У������ķ�Ӧ����ȼ�շ�Ӧ
D����ͼ�У�ʪ�����ɫ��������ɫ����������Һ�����ձ��У�����Һ�����ԣ������Cl2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 25��ʱ������c(CH3COOH)+c(CH3COO-)=0��1mol��L-1�Ĵ���ʹ����ƻ����Һ�У�c(CH3COO-)��pH�Ĺ�ϵ��ͼ��ʾ�����������������
25��ʱ������c(CH3COOH)+c(CH3COO-)=0��1mol��L-1�Ĵ���ʹ����ƻ����Һ�У�c(CH3COO-)��pH�Ĺ�ϵ��ͼ��ʾ�����������������
A�����¶��´���ĵ��볣��KaΪl0-4.75mol��L-l
B��M������ʾ����Һ�У�
c(Na+)+c(H+)+c(CH3COOH)=0��1 mol��L-1
C��N������ʾ����Һ�У�
c(CH3COO-)>c(CH3COOH)>c(H+)>c(OH-)
D��Q������ʾ����Һ�м���������0��05mol��L-1NaOH��Һ��ַ�Ӧ��pH>7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�ӦaA(g)��bB(g)  pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����
pC(g)���ﵽƽ��������¶�ʱ��B��ת���ʱ����Сѹǿʱ�������ϵ��C����������Ҳ��С����
(1)�÷�Ӧ���淴Ӧ��________�ȷ�Ӧ����a��b________p(�>����<������)��
(2)��ѹʱ��A����������________(�������С�����䡱����ͬ)������Ӧ����________��
(3)������B(�������)����A��ת����________��B��ת����________��
(4)�������¶ȣ���ƽ��ʱ��B��C��Ũ��֮��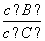 ��________��
��________��
(5)�����������ƽ��ʱ��������������ʵ���________��
(6)��B����ɫ����,A��C��Ϊ��ɫ����,�����C(�������)ʱ��������ɫ________����ά�������������ѹǿ����,��������ʱ,��������ɫ________��(���dz����������䡱)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com