
����˵������ȷ����(����)
A�������ԭˮ����ȵ��ˮ��������ܻ�����������
B��������ˮ(��NH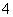 ��NH3)���û�ѧ��������绯ѧ����������
��NH3)���û�ѧ��������绯ѧ����������
C��ij�ֻ�ѧ��⼼�����м��ߵ������ȣ��ɼ�����ϸ��(V��10��12 L)�ڵ�����Ŀ����ӣ��ݴ˿�����ü�⼼���ܲ���ϸ����Ũ��ԼΪ10��12��10��11mol·L��1��Ŀ�����
D�������������Ӽ״��û��ȼ�ϵ���ֵ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(N2H4)�ǻ����������ȼ�ϣ�����N2O4��Ӧʱ��N2O4Ϊ�����������ɵ�����ˮ��������֪��N2(g)��2O2(g)===N2O4(g)����H����8.7 kJ/mol��N2H4(g)��O2(g)===N2(g)��2H2O(g)����H����534.0 kJ/mol�����б�ʾ�¸�N2O4��Ӧ���Ȼ�ѧ����ʽ����ȷ����(����)
A��2N2H4(g)��N2O4(g)===3N2(g)��4H2O(g) ��H����542.7 kJ/mol
B��2N2H4(g)��N2O4(g)===3N2(g)��4H2O(g) ��H����1 059.3 kJ/mol
C��2N2H4(g)��N2O4(g)===3N2(g)��4H2O(g) ��H����1 076.7 kJ/mol
D��N2H4(g)�� N2O4(g)===
N2O4(g)=== N2(g)��2H2O(
N2(g)��2H2O( g) ��H����1 076.7 kJ/mol
g) ��H����1 076.7 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ҩ�����е���Ҫ���Գɷ�֮һ�����п������Ϳ��������á��������Ҳ��ͨ����ͼ��ʾ�����ϳɣ�

�ش��������⣺
��1���������ķ���ʽΪ�� �� ����1 mol��������ȫȼ������������ mol O2.
��2���������ĺϳɷ���Ϊ��
|
 ��CHO��(CH3CO)2O ��
��CHO��(CH3CO)2O ��
 ��CH��CHCOOH�� A
��CH��CHCOOH�� A
 |
|


 ��CH��CHCOOH ���� �������
��CH��CHCOOH ���� ������� ��֪�ںϳ������Ļ�ѧ��Ӧ�У���Ӧ������ʵ���֮��Ϊ1︰1��������A���뱥��NaHCO3��Һ��Ӧ�������壬��������A�Ľṹ��ʽ�������� ��
��3����Ӧ�ٵķ�Ӧ����Ϊ�������� ���������͢�Ӧ�����Եõ�һ���������ɸ����ķ�Ӧ����ʽΪ ��������ע����Ӧ������
��4�����й��ڻ������������˵����ȷ���� ������ţ���
a�������ڷ��㻯����
b��������H2�����ӳɷ�Ӧ�������ĵ�H2����
c������ʹ����KMnO4��Һ��ɫ
d��������FeCl3��Һ������ɫ��Ӧ
��5���������������Ļ�������ͬ���칹����Ľṹ��ʽΪ
����FeCl3��Һ��ʾ��ɫ
��l mol ����Ũ��ˮ��Ӧ���������1 mol Br2
�ۢ��ĺ˴Ź�������������壬�����֮��Ϊ1︰1︰2︰2︰6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ҫ����д�йصĵ缫��Ӧʽ���ܷ���ʽ��
(1)�ö��Ե缫���AgNO3��Һ��
������Ӧʽ_____________________________________________________��
������Ӧʽ_____________________________________________________��
�ܷ�Ӧ���ӷ���ʽ________________________________________________��
(2)�ö��Ե缫���MgCl2��Һ
������Ӧʽ______________________________________________________��
������Ӧʽ______________________________________________________��
�ܷ�Ӧ���ӷ���ʽ________________________________________________��
(3)�������缫���NaCl��Һ
������Ӧʽ______________________________________________________��
������Ӧʽ______________________________________________________��
�ܻ�ѧ����ʽ____________________________________________________��
(4)��ͭ���缫���������Һ
������Ӧʽ_____________________________________________________��
������Ӧʽ_____________________________________________________��
�ܷ�Ӧ���ӷ���ʽ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijͬѧ��װ����ͼ��ʾ�ĵ绯ѧװ�ã��缫��ΪAl�������缫��ΪCu����(����)
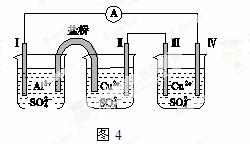
A�������� �缫������
�缫������ �����缫��
�����缫��
B���缫������ԭ��Ӧ
C���缫�����ܽ�
D���缫��ĵ缫��Ӧ��Cu2����2e��===Cu
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ת������Դ���úͻ�����������Ҫ�о����⡣�������������ж��ַ�����
(1)���ռ�����H2S�����Һ���뵽��ͼ��ʾ�ĵ��ص����������е�⡣���������������������·�Ӧ��
S2����2e��===S��(n��1)S��S2��===S

��д�����ʱ�����ĵ缫��Ӧʽ��________________��
�ڵ�������������Һ��ϡ�����ữ�õ����ʣ������ӷ���ʽ��д��__________________________��
(2)��H2S�Ϳ����Ļ������ͨ��FeCl3��FeCl2��CuCl2�Ļ����Һ�з�Ӧ����S��������ת����ͼ��ʾ��

����ͼʾ ��ת���У����ϼ�
��ת���У����ϼ� �����Ԫ����________��
�����Ԫ����________��
�ڷ�Ӧ�е���1 m ol H2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬������O2�����ʵ���Ϊ________��
ol H2Sת��Ϊ����ʱ��������Һ��Fe3�������ʵ������䣬������O2�����ʵ���Ϊ________��
�����¶�һ���Ͳ�������Һ�������£�����ͨ�������壬����ֽ��衣��ʹ���ɵ������в���CuS���ɲ�ȡ�Ĵ�ʩ��________________��
(3)H2S�ڸ����·ֽ�������������H2������Ӧ�ڲ�ͬ�¶��´ﵽƽ��ʱ����������и���ֵ����������ͼ��ʾ��H2S�ڸ����·ֽⷴӦ�Ļ�ѧ����ʽΪ__________________________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������ڳ��ͷŵ�ʱ�����ķ�ӦΪ��
Fe��NiO2��2H2O Fe(OH)2��Ni(OH)2������˵������ȷ����(����)
Fe(OH)2��Ni(OH)2������˵������ȷ����(����)
A���ŵ�ʱ�������Ϸ�����Ӧ��������Fe
B���ŵ�ʱ��������Ӧ�ǣ�NiO2��2e����2H��===Ni(OH)2
C�����ʱ��������Ӧ�ǣ�N i(OH)2��2e����2OH��===NiO2��2H2O
i(OH)2��2e����2OH��===NiO2��2H2O
D�����ʱ����������pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���о��л�����ʱ������16 8O��Ϊʾ��ԭ�ӡ�16 8O��ԭ�Ӻ�����������
A��8 B��18 C��10 D��28
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������������ǿ���Ƚ���ȷ����
A��H2SO3>H2SO4 B��HNO3>H3PO4 C��HClO>HClO2 D��H2CO3>HNO3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com