
£Ø12·Ö£©£Ø1£©ŅŃÖŖŌŚ25”ꏱ£¬“×Ėį”¢Ģ¼ĖįŗĶŃĒĮņĖįµÄµēĄėĘ½ŗā³£Źż·Ö±šĪŖ£ŗ
“×Ėį Ki = 1.75”Į10£5
Ģ¼Ėį Ki 1= 4.30”Į10£7 Ki 2 = 5.61”Į10£11
ŃĒĮņĖį Ki 1= 1.54”Į10£2 Ki 2 = 1.02”Į10£7
Š“³öĢ¼ĖįµÄµŚŅ»¼¶µēĄėĘ½ŗā³£Źż±ķ“ļŹ½£ŗKi = _ __
ŌŚĻąĶ¬Ģõ¼žĻĀ£¬ŹŌ±Č½ĻH2CO3”¢HCO3£ŗĶHSO3£µÄĖįŠŌ×īĒæµÄŹĒ
¢Ū Čō±£³ÖĪĀ¶Č²»±ä£¬ŌŚ“×ĖįČÜŅŗÖŠ¼ÓČėÉŁĮæŃĪĖį£¬ĻĀĮŠĮæ»į±äŠ”µÄŹĒ £ØĢīŠņŗÅ£©
A. c(CH3COO£) B. c(H+) C. w“×ĖįµēĄėĘ½ŗā³£Źż D. “×ĖįµÄµēĄė¶Č  £Ø2£©Ņ»¶ØĪĀ¶ČĻĀµÄÄŃČܵē½āÖŹAmBnŌŚĖ®ČÜŅŗÖŠ“ļµ½³ĮµķČܽāĘ½ŗāŹ±£¬ĘäĘ½ŗā³£Źż
£Ø2£©Ņ»¶ØĪĀ¶ČĻĀµÄÄŃČܵē½āÖŹAmBnŌŚĖ®ČÜŅŗÖŠ“ļµ½³ĮµķČܽāĘ½ŗāŹ±£¬ĘäĘ½ŗā³£Źż
Ksp£½cm(An£«)”Įcn(Bm£)£¬³ĘĪŖÄŃČܵē½āÖŹµÄĄė×Ó»ż”£ŌŚ25”ꏱ£¬AgClµÄ°×É«Šü×ĒŅŗÖŠ£¬ŅĄ“Ī¼ÓČėµČÅØ¶ČµÄKIČÜŅŗŗĶNa2SČÜŅŗ£¬¹Ū²ģµ½µÄĻÖĻóŹĒĻČ³öĻÖ»ĘÉ«³Įµķ£¬×īŗóÉś³ÉŗŚÉ«³Įµķ”£
ĻĀĮŠŠšŹö²»ÕżČ·µÄŹĒ  w.w.w.zxxk.c.o.m
w.w.w.zxxk.c.o.m
A£®ČܶȻżŠ”µÄ³ĮµķæÉŅŌ×Ŗ»ÆĪŖČܶȻżøüŠ”µÄ³Įµķ
B£®ČōĻČ¼ÓČėNa2SČÜŅŗ£¬ŌŁ¼ÓČėKClČÜŅŗ£¬ŌņĪŽ°×É«³Įµķ²śÉś
C£®25”ꏱ£¬±„ŗĶAgCl”¢AgI”¢Ag2SČÜŅŗÖŠĖłŗ¬Ag£«µÄÅضČĻąĶ¬
D£®25”ꏱ£¬AgCl¹ĢĢåŌŚµČĪļÖŹµÄĮæÅØ¶ČµÄNaCl”¢CaCl2ČÜŅŗÖŠµÄČܶȻżĻąĶ¬
£Ø3£©³£ĪĀĻĀ£¬Č”pH=2µÄŃĪĖįŗĶ“×ĖįČÜŅŗø÷100mL£¬ĻņĘäÖŠ·Ö±š¼ÓČėŹŹĮæµÄZnĮ££¬·“Ó¦¹ż³ĢÖŠĮ½ČÜŅŗµÄpH±ä»ÆČēĶ¼ĖłŹ¾”£ŌņĶ¼ÖŠ±ķŹ¾“×ĖįČÜŅŗÖŠpH±ä»ÆĒśĻߵďĒ £ØĢī”°A”±»ņ”°B”±£©”£
ÉčŃĪĖįÖŠ¼ÓČėµÄZnÖŹĮæĪŖm1£¬ “×ĖįČÜŅŗÖŠ¼ÓČėµÄZnÖŹĮæĪŖm2”£Ōņm1 m2£ØŃ”Ģī”°£¼”±”¢”°=”±”¢”°£¾”±£©
£Ø1£©¢Ł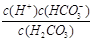 ¢Ś H2CO3 ¢Ū A D £Ø2£©C
¢Ś H2CO3 ¢Ū A D £Ø2£©C
£Ø3£©B£¬<
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©¢ŁŠ“³öĢ¼ĖįµŚŅ»²½µēĄėµÄµēĄė·½³ĢŹ½£¬øł¾ŻµēĄėĘ½ŗā³£ŹżµÄ±ķ“ļŹ½µĆKi£»¢ŚµēĄėĘ½ŗā³£ŹżŌ½“ó£¬ĖįŠŌŌ½Ē棬Ģ¼ĖįµÄĖįŠŌ擵ŚŅ»²½µēĄėµÄĘ½ŗā³£Źż£¬HSO3£µÄĖįŠŌæ“ŃĒĮņĖįµÄµŚ¶ž²½µēĄėµÄĘ½ŗā³£ŹżKi2£»¢Ū¼ÓČėŃĪĖį£¬ĒāĄė×ÓÅضČŌö“󣬓×ĖįµÄµēĄėĘ½ŗāĻņ×óŅĘ¶Æ£¬µēĄė³Ģ¶Č¼õŠ”£¬“×ĖįøłĄė×ÓµÄÅØ¶Č¼õŠ”£¬ĒāĄė×ÓµÄÅضČŌö“󣬵ēĄėĘ½ŗā³£ŹżÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬¹ŹµēĄėĘ½ŗā³£Źż²»±ä£¬¹ŹŃ”AD£»£Ø2£©A”¢ÄŃČܵÄæÉŅŌ×Ŗ»Æ³ÉøüÄŃČܵģ¬¹ŹAÕżČ·£»B”¢ĻČÉś³ÉĮĖĮņ»ÆŅų³Įµķ£¬ŅņĀČ»ÆŅųµÄČܽāÄÜĮ¦“󣬹Ź²»ÄÜ×Ŗ»Æ³ÉĀČ»ÆŅų³Įµķ£¬¹ŹBÕżČ·£»ŅņČżÕßµÄKsp²»ĻąĶ¬£¬¼“ČܽāÄÜĮ¦²»ĻąĶ¬£¬¹ŹČżÕߵı„ŗĶČÜŅŗÖŠµÄŅųĄė×ÓµÄÅØ¶Č²»ĻąĶ¬£¬¹ŹC“ķĪó£»D”¢ČܶȻżÖ»ÓėĪĀ¶ČÓŠ¹Ų£¬ÓėŌŚÄÄÖÖČÜŅŗÖŠĪŽ¹Ų£¬¹ŹŌŚĮ½ÖÖČÜŅŗÖŠµÄČܶȻżĻąĶ¬£¬¹ŹDÕżČ·£»¹ŹŃ”C£»£Ø3£©æŖŹ¼Ź±Į½ČÜŅŗÖŠµÄĒāĄė×ÓÅضČĻąµČ£¬“×ĖįČÜŅŗÖŠ»¹“ęŌŚ“×ĖįµÄµēĄėĘ½ŗā£¬Ėę×Å·“Ó¦µÄ½ųŠŠ£¬ĒāĄė×ÓµÄĻūŗÄ£¬“×ĖįµÄµēĄėĘ½ŗāĻņÓŅŅĘ¶Æ£¬Ź¹ĒāĄė×ÓµÄÅضČŌö“󣬹Ź“×ĖįČÜŅŗÖŠµÄĒāĄė×ÓÅØ¶Č¼õŠ”µÄĀż£¬¹ŹBĒśĻߏĒ“×Ėį£»ŌŚpHĻąµČ£¬¼“æŖŹ¼ĒāĄė×ÓÅضČĻąµČµÄĢõ¼žĻĀ£¬“×ĖįµÄĪļÖŹµÄĮæÅØ¶Č“ó£¬¹ŹŌŚĢå»żĻąµČµÄĢõ¼žĻĀ£¬“×ĖįĻūŗÄµÄŠæ¶ą”£
æ¼µć£ŗµēĄėĘ½ŗā³£ŹżµÄ±ķ“ļŹ½”¢µēĄėĘ½ŗāµÄÓ°ĻģŅņĖŲ”¢ČܽāĘ½ŗāµÄÓ°ĻģŅņĖŲ”¢ČܶȻżµÄÓ°ĻģŅņĖŲµČÖŖŹ¶”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ŹŅĪĀĻĀ£¬Ä³ÅØ¶ČµÄ“×ĖįČÜŅŗÖŠn(CH3COO£)=0.01mol£¬ĻĀĮŠŠšŹöÕżČ·µÄŹĒ
| A£®ĻņøĆ“×ĖįČÜŅŗÖŠ¼ÓČėµČĢå»żµČÅØ¶ČµÄŃĪĖį£¬ŅÖÖĘ“×ĖįµÄµēĄė£¬ČÜŅŗÖŠc(H+)Ōö“ó |
| B£®Čō“×ĖįČÜŅŗĢå»żĪŖ1L£¬Ōņc(CH3COOH)=0.01mol/L |
| C£®ÓėNaOHĒ”ŗĆÖŠŗĶŹ±£¬ČÜŅŗÖŠc(Na+)£¼c(CH3COO£) |
| D£®ÓėµČĢå»żµČÅØ¶ČµÄ“×ĖįÄĘČÜŅŗ»ģŗĻ£¬ČÜŅŗÖŠc(Na+)+ c(H+)=c(CH3COO£)+ c(OH£) |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠĖµ·ØÖŠ“ķĪóµÄŹĒ
| A£®0.5 L 2 mol”¤L£1 AlCl3ČÜŅŗÖŠ£¬Al3£«ŗĶCl£×ÜŹżŠ”ÓŚ4”Į6.02”Į1023øö |
| B£®ÖĘ³É0.5 L 10 mol”¤L£1µÄŃĪĖį£¬ŠčŅŖ±ź×¼×“æöĻĀµÄĀČ»ÆĒāĘųĢå112 L |
| C£®“Ó1 L 1 mol”¤L£1µÄNaClČÜŅŗÖŠŅĘČ”³ö10 mLČÜŅŗ£¬ĘäÅØ¶ČŹĒ1 mol”¤L£1 |
| D£®10 g 98%µÄĮņĖį(ĆܶČĪŖ1.84 g”¤cm£3)Óė10 mL18.4 mol”¤L£1µÄĮņĖįµÄÅØ¶Č²»Ķ¬ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĻÖÓŠŹŅĪĀĻĀÅØ¶Č¾łĪŖ1”Į10-3mol/LµÄ¼øÖÖČÜŅŗ£ŗ
¢ŁŃĪĖį”¢¢ŚĮņĖį”¢¢Ū“×Ėį”¢¢ÜĀČ»Æļ§”¢¢Ż°±Ė®”¢¢ŽNaOH£¬»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©½«¢Ū”¢¢Ž»ģŗĻŗó£¬ČōČÜŅŗ³ŹÖŠŠŌ£¬ŌņĻūŗÄĮ½ČÜŅŗµÄĢå»żĪŖ¢Ū ________ ¢Ž£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©ČÜŅŗÖŠµÄĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ
£Ø2£©½«µČĢå»żµÄ¢Ł”¢¢Ż»ģŗĻ£¬ŌņČÜŅŗµÄpH ________ 7£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©
ÓĆĄė×Ó·½³ĢŹ½ĖµĆ÷ĘäŌŅņ _______________________
£Ø3£©ĻņĻąĶ¬Ģå»żµÄ¢Ł”¢¢Ś”¢¢ŪČÜŅŗÖŠ·Ö±š¼ÓČėĻąĶ¬µÄĒŅ×ćĮæµÄŠæĮ££¬·“Ó¦µÄ³õŹ¼ĖŁĀŹÓÉæģµ½ĀżµÄĪŖ________ £ØĢīŠ“ŠņŗÅ£©
×īÖÕ²śÉśH2×ÜĮæµÄ¹ŲĻµĪŖ___________£ØĢīŠ“ŠņŗÅ£©
£Ø4£©ĻņĻąĶ¬Ģå»żµÄ¢Ł”¢¢ŪČÜŅŗÖŠ·Ö±š¼ÓČėĻąĶ¬ÅØ¶Č”¢ĻąĶ¬Ģå»żµÄCH3COONaČÜŅŗ£¬³ä·Ö»ģŗĻŗ󣬻ģŗĻŅŗµÄpH “󊔹ŲĻµĪŖ¢Ł _________ ¢Ū£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©
£Ø5£©½«µČĢå»żµÄ¢Ż”¢¢ŽČÜŅŗ¼ÓČČÖĮĶ¬ĪĀ¶Čŗó£¬ČÜŅŗµÄpH ¢Ż _____ ¢Ž£ØĢī”°>”±”¢”°<”±»ņ”°=”±£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø1£©½«øĆĪĀ¶ČĻĀa mL pH="13" NaOHČÜŅŗÓėb mL0.05mol/L H2SO4»ģŗĻ£¬ĖłµĆ»ģŗĻČÜŅŗµÄpH=7£¬
Ōņa:b =
£Ø2£©ČōŌŚŗ¬ÓŠ×ćĮæAgCl¹ĢĢåµÄ±„ŗĶAgClČÜŅŗÖŠ·Ö±š·ÅČė£ŗ
| A£®100mLÕōĮóĖ®ÖŠ£» | B£®100mL 0.2 mol”¤L£1AgNO3ČÜŅŗÖŠ£» |
| C£®100 mL 0.1 mol”¤L£1AlCl3ČÜŅŗÖŠ£» | D£®100mL 0.1 mol”¤L£1ŃĪĖįČÜŅŗÖŠ”£ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©¹¤ŅµÉĻĪŖĮĖ²ā¶Ø»ŌĶæó£ØÖ÷ŅŖ³É·ÖŹĒCu2S£©ÖŠCu2SµÄÖŹĮæ·ÖŹż£¬Éč¼ĘĮĖČēĶ¼×°ÖĆ”£ŹµŃ鏱°“ČēĻĀ²½Öč²Ł×÷£ŗ
| A£®Į¬½ÓŗĆŅĒĘ÷£¬Ź¹Ęä³ÉĪŖČēĶ¼×°ÖĆ£¬²¢¼ģ²é×°ÖƵÄĘųĆÜŠŌ”£ |
| B£®³ĘȔъĻøµÄ»ŌĶæóѳʷ1.000g”£ |
| C£®½«³ĘĮæŗƵÄѳʷŠ”ŠÄµŲ·ÅČėÓ²ÖŹ²£Į§¹ÜÖŠ”£ |
| D£®ŅŌĆæ·ÖÖÓ1LµÄĖŁĀŹ¹ÄČėæÕĘų”£ |
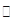 SO2 +2Cuӣ
SO2 +2Cuӣ
| µĪ¶Ø “ĪŹż | “ż²āČÜŅŗµÄ Ģå»ż/mL | ±ź×¼ČÜŅŗµÄĢå»ż | |
| µĪ¶ØĒ°æĢ¶Č/mL | µĪ¶ØŗóæĢ¶Č/mL | ||
| 1 | 25.00 | 1.04 | 21.03 |
| 2 | 25.00 | 1.98 | 21.99 |
| 3 | 25.00 | 3.20 | 21.24 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
¶Ō½šŹōÖĘĘ·½ųŠŠæ¹øÆŹ““¦Ąķ£¬æÉŃÓ³¤ĘäŹ¹ÓĆŹŁĆü”£
£Ø1£©ŅŌĻĀĪŖĀĮ²Ä±ķĆꓦĄķµÄŅ»ÖÖ·½·Ø£ŗ
¢Ł ¼īĻ“µÄÄæµÄŹĒ³żČ„ĀĮ²Ä±ķĆęµÄ×ŌČ»Ńõ»ÆĤ£¬ĪŖ½«¼īĻ“²ŪŅŗÖŠĀĮŅŌ³ĮµķŠĪŹ½»ŲŹÕ£¬×īŗĆĻņ²ŪŅŗÖŠ¼ÓČėĻĀĮŠŹŌ¼ĮÖŠµÄ ”£
a£®NH3 b£®CO2 c£®NaOH d£®HNO3
¢ŚŅŌĀĮ²ÄĪŖŃō¼«£¬ŌŚH2SO4ČÜŅŗÖŠµē½ā£¬ĀĮ²Ä±ķĆęŠĪ³ÉŃõ»ÆĤ£¬Ńō¼«µē¼«·“Ó¦ĪŖ ”£Č”ÉŁĮæ·Ļµē½āŅŗ£¬¼ÓČėNaHCO3£¬ČÜŅŗŗó²śÉśĘųÅŻŗĶ°×É«³Įµķ£¬²śÉś³ĮµķµÄŌŅņŹĒ ”£
£Ø2£©¶ĘĶæÉ·ĄÖ¹ĢśÖĘĘ·øÆŹ“£¬µē¶ĘŹ±ÓĆĶ¶ų²»ÓĆŹÆÄ«×÷Ńō¼«µÄŌŅņŹĒ ”£
£Ø3£©ĄūÓĆĻĀĶ¼×°ÖĆ£¬æÉŅŌÄ£ÄāĢśµÄµē»Æѧ·Ą»¤”£ČōXĪŖĢ¼°ō£¬ĪŖ¼õ»ŗĢśµÄøÆŹ“£¬æŖ¹ŲKÓ¦ÖĆÓŚ “¦”£ČōXĪŖŠæ£¬æŖ¹ŲKÖĆÓŚ “¦”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ÓŠČĖŌų½ØŅéÓĆAG±ķŹ¾ČÜŅŗµÄĖį¶Č£Øacidity grade£©£¬AGµÄ¶ØŅåĪŖ
AG=lg£Øc£ØH£«£©/c£ØOH££©£©”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚ25”ꏱ£¬ČōČÜŅŗ³ŹÖŠŠŌ£¬ŌņAG= ”£
£Ø2£©ŌŚ25”ꏱ£¬ČōČÜŅŗµÄpH=12£¬ŌņAG= ”£
£Ø3£©ŌŚ25”ꏱ£¬ČÜŅŗµÄpHÓėAGµÄ»»Ėć¹«Ź½ĪŖAG= £ØŅŖ»Æ¼ņ£©”£
£Ø4£©ŌŚ25”ꏱ£¬½«AG=-12µÄNaOHČÜŅŗaLÓėAG=12µÄH2SO4ČÜŅŗbL»ģŗĻ£Ø»ģŗĻŗóµÄĢå»żĪó²īæÉŅŌ ŗöĀŌ£©£¬ČōĖłµĆ»ģŗĻŅŗµÄAG=10£¬Ōņa”Ćb= ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²āŃŖøʵÄŗ¬ĮæŹ±,æɽ«2.0 mLŃŖŅŗÓĆÕōĮóĖ®Ļ”ŹĶŗó,ĻņĘäÖŠ¼ÓČė×ćĮæ²ŻĖįļ§(NH4)2C2O4¾§Ģå,·“Ӧɜ³ÉCaC2O4³Įµķ”£½«³ĮµķÓĆĻ”ĮņĖį“¦ĄķµĆH2C2O4ŗó,ŌŁÓĆĖįŠŌKMnO4ČÜŅŗµĪ¶Ø,Ńõ»Æ²śĪļĪŖCO2,»¹Ō²śĪļĪŖMn2+,ČōÖÕµćŹ±ÓĆČ„20.0 mL 1.0”Į10-4 mol”¤L-1µÄKMnO4ČÜŅŗ”£
(1)Š“³öÓĆKMnO4µĪ¶ØH2C2O4µÄĄė×Ó·½³ĢŹ½”””””””””””””””””””””””””””””””””””£
(2)ÅŠ¶ĻµĪ¶ØÖÕµćµÄ·½·ØŹĒ”””””””””””””£
(3)¼ĘĖć:ŃŖŅŗÖŠŗ¬øĘĄė×ÓµÄÅضČĪŖ””””””””g”¤mL-1”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com