
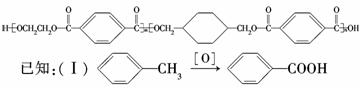
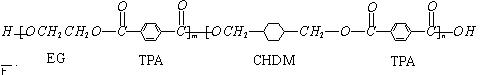
ĪŅ¹śµŚ¶ž“śÉķ·ŻÖ¤²ÉÓƵďĒ¾ßÓŠĀĢÉ«»·±£ŠŌÄܵÄPETGŠĀ²ÄĮĻ£¬PETGŠĀ²Ä
ĮĻæÉŅŌ»ŲŹÕŌŁĄūÓĆ£¬¶ųĒŅ¶ŌÖܱ߻·¾³²»¹¹³ÉČĪŗĪĪŪČ¾£®PETGµÄ½į¹¹¼ņŹ½ĪŖ£ŗ
 |
(¢ņ)RCOOR”䣫R”åOHØD”śRCOOR”士R”äOH(R”¢R”äR”å±ķŹ¾Ģž»ł)
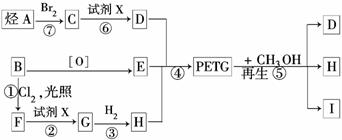 ÕāÖÖ²ÄĮĻæɲÉÓĆŅŌĻĀĀ·ĻßŗĻ³É£ŗ
ÕāÖÖ²ÄĮĻæɲÉÓĆŅŌĻĀĀ·ĻßŗĻ³É£ŗ
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)·“Ó¦¢Ś¢Ž¼ÓČėµÄŹŌ¼ĮXŹĒ________£¬·“Ó¦¢Ł”«¢ßÖŠŹōÓŚČ”“ś·“Ó¦µÄŹĒ________£®
(2)Š“³öĪļÖŹIµÄ½į¹¹¼ņŹ½£ŗ____________________________________________.
(3)Š“³ö·“Ó¦¢ŽµÄ»Æѧ·½³ĢŹ½£ŗ___________________________________________
______________________________________________________________________.
(4)ŗĻ³ÉPETGŹ±ø÷µ„ĢåµÄĪļÖŹµÄĮæµÄ±ČĄż¹ŲĻµŹĒ£ŗ
n(D)”Ćn(E)”Ćn(H)£½________(ÓĆm”¢n±ķŹ¾)£®

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ









| NaOH |
| ”÷ |
| NaOH |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŅ¹śµŚ¶ž“śÉķ·ŻÖ¤²ÉÓƵďĒ¾ßÓŠĀĢÉ«»·±£ŠŌÄܵÄPETGŠĀ²ÄĮĻ£¬PETGŠĀ²ÄĮĻæÉŅŌ»ŲŹÕŌŁĄūÓĆ£¬¶ųĒŅ¶ŌÖܱ߻·¾³²»¹¹³ÉČĪŗĪĪŪČ¾”£ÕāŅ»²ÄĮĻŹĒÓÉ»ŖŠÅĖÜŅµ·¢Õ¹ÓŠĻŽ¹«Ė¾×īŠĀŃŠ·¢³É¹¦µÄŠĀ²ÄĮĻ£¬²¢³ÉĪŖ¹«°²²æ¶Øµć²śµŲ”£PETGµÄ½į¹¹¼ņŹ½ĪŖ£ŗ
ĻĀĮŠÅŠ¶ĻÕżČ·µÄŹĒ £Ø £©
A”¢PETGŹĒÓŠ»śøß·Ö×Ó»ÆŗĻĪļ B”¢PETGŹĒĶعż¼Ó¾Ū·“Ӧɜ²śµÄ
C”¢øĆĪļÖŹŅ×ČܽāÓŚĖ® D”¢øĆĪļÖŹŹĒĢåŠĶ½į¹¹µÄøß·Ö×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗÓŠ»ś»Æѧ»ł“”²āŹŌŃé ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©ĪŅ¹śµŚ¶ž“śÉķ·ŻÖ¤²ÉÓƵďĒ¾ßÓŠĀĢÉ«»·±£ŠŌÄܵÄPETGŠĀ²ÄĮĻ£¬PETGŠĀ²ÄĮĻæÉŅŌ»ŲŹÕŌŁĄūÓĆ£¬¶ųĒŅ¶ŌÖܱ߻·¾³²»¹¹³ÉČĪŗĪĪŪČ¾”£
PETGµÄ½į¹¹¼ņŹ½ĪŖ£ŗ
ŅŃÖŖ£ŗ
£Ø2£©RCOOR1+R2OH RCOOR2+R1OH£ØR”¢R1”¢R2“ś±ķĢž»ł£©
RCOOR2+R1OH£ØR”¢R1”¢R2“ś±ķĢž»ł£©
ÕāÖÖ²ÄĮĻæɲÉÓĆĻĀĮŠŗĻ³ÉĀ·Ļߣŗ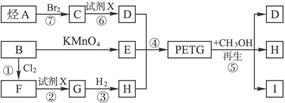
ŹŌĶź³ÉĻĀĮŠĪŹĢā£ŗ
£Ø1£©·“Ó¦¢Ś¢Ž¼ÓČėµÄŹŌ¼ĮXŹĒ______________________________________”£
£Ø2£©¢ŻµÄ·“Ó¦ĄąŠĶŹĒ________________________________________”£
£Ø3£©Š“³ö½į¹¹¼ņŹ½£ŗB.____________________________________£¬I._________”£
£Ø4£©ŗĻ³ÉŹ±Ó¦æŲÖʵ„ĢåµÄĪļÖŹµÄĮæ£ŗ
n£ØD£©”Ćn£ØE£©”Ćn£ØH£©=________”Ć________”Ć________£ØÓĆm”¢n±ķŹ¾£©”£
£Ø5£©Š“³ö»Æѧ·½³ĢŹ½£ŗ
·“Ó¦¢Ū____________________£¬·“Ó¦¢Ž____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013Äźŗ£ÄĻĒķŗ£¼Ī»żÖŠŃ§ø߶žÉĻøßÖŠ½Ģѧ֏Įæ¼ą²āĄķ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗµ„Ń”Ģā
ĪŅ¹śµŚ¶ž“śÉķ·ŻÖ¤²ÉÓƵďĒ¾ßÓŠĀĢÉ«»·±£ŠŌÄܵÄPETGŠĀ²ÄĮĻ£¬PETGŠĀ²ÄĮĻæÉŅŌ»ŲŹÕŌŁĄūÓĆ£¬¶ųĒŅ¶ŌÖܱ߻·¾³²»¹¹³ÉČĪŗĪĪŪČ¾”£PETGµÄ½į¹¹¼ņŹ½ĪŖ£ŗ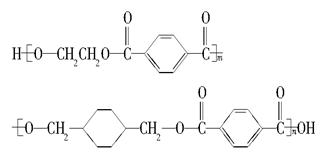
ŗĻ³ÉPETGµÄµ„ĢåÓŠ(””””)
| A£®2ÖÖ”” | B£®3ÖÖ | C£®4ÖÖ | D£®5ÖÖ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com