
A��B��C��D��E��F�������������±仯��ϵ��E�ǵ���ɫ��ĩ���жϣ�
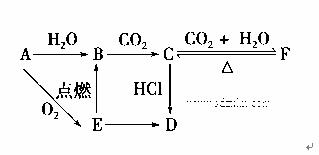
(1)д��A��B��C��D��E��F�Ļ�ѧʽ��
B.________��C.________��D.________��E��________��
(2)д���йط�Ӧ�Ļ�ѧ����ʽ(�����ӷ�Ӧ��ֱ��д���ӷ���ʽ)
E��B�� ______________________________________________ _ ��
C��F�� ____________________________________________________��
F��C�� ____________________________________________________��
(3)����ʵ�鷽���У�����ȷ�ⶨNa2CO3��NaHCO3�������Na2CO3������������
A. ȡa�˻�����ּ��ȣ�����b��
B. ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b�˹���
C. ȡa�˻����������ϡ�����ַ�Ӧ���ݳ�����ֱ���ü�ʯ�����գ�����b��
D. ȡa�˻����������Ba(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��塣

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��ش��������⣺
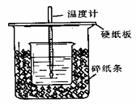 ��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ��
��1����ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ��
���������� ��
��2���ձ���������ֽ���������� ��
��3�����ձ����粻��Ӳֽ�壬��õ��к�����ֵ (ƫ��ƫС����Ӱ��)
��4�������60mL0.50mol/L������50mL0.55mol/LNaOH��Һ���з�Ӧ��������ʵ����ȣ����ų������� (���ȡ�����ȡ�)�������к��� (���ȡ�����ȡ�)����������
��5������ͬŨ�Ⱥ�����İ�ˮ(NH3��H2O)����NaOH��Һ��������ʵ�飬��õ��к��ȵ���ֵ�� �����ƫ����ƫС��������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������������п��a��b���ֱ��������ʢ������ϡ������ձ��У��������a���ձ����ټ���һ������CuO��ĩ�����и�ͼ��ʾ�������V��H2���뷴Ӧʱ��t�Ĺ�ϵ��������ȷ���ǣ�������
| �� | A�� |
| B�� |
| C�� |
| D�� |
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��ȤС���ڿ����У���ijһ����Һ�ɷ�(��֪����������ԭ������)�����˼�⣬�������μ�������±���ʾ
| ������ | ��Һ�м������������ |
| ��һ�� | KCl��K2SO4��Na2CO3��NaCl |
| �ڶ��� | KCl��AlCl3��Na2SO4��K2CO3 |
| ������ | Na2SO4��KCl��K2CO3��NaCl |
������˵����������
A�����μ��������ȷ
B������Һ�е����������ж�
C��Ϊ�˼���SO42-��Ӧ�ȼӹ���ϡ������ٵμ�Ba(NO3)2���۲��Ƿ��г�������
D��Ϊ��ȷ���Ƿ����CO32-�����������еμ�CaCl2��Һ���۲��Ƿ��г�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����Ƽ��仯�����˵����ȷ����
���ƼغϽ�ͨ��״���³�Һ̬������ԭ�ӷ�Ӧ�ѵĵ��ȼ�
���ƵĻ�ѧ���ʻ��ã��������ƿɱ������л��ܼ�CCl4��
�����ڿ����л�����������ȼ����������ȼ�ո�Ϊ����
�������ƱȽϻ��ã��������ܴ�����Һ���û����������˳������ƺ���Ľ���
����������ijЩ��������������Ʊ�����
��Na2CO3��Һ�ܸ�����Һ��Ӧ�������ܸ�����Һ��Ӧ
A���٢ڢۢ� B���ڢۢ�
C���٢� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������������ϡ���ᷴӦ���ų�NO���ʵ���������
A��FeO B��Fe2O3 C��FeSO4 D��Fe3O4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й���Һ��ɵ�������������
A����ˮ�������c(H��)��10��13mol/L����Һ�п��ܴ��ڣ�HCO3����K+��Na+��Cl��
B��������Һ�п��ܴ�������Na+��ClO ��SO42-��I
��SO42-��I
C������0.1 mol/L Fe3+ ����Һ�п��Դ������ڣ�K+��Mg2+��I-��NO3-
D�������£�pH=l����Һ��һ�����Դ��ڣ�Na+��Fe3+��NO3-��SO42-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�йغϳɰ���ҵ������˵���У���ȷ����(����)
A���Ӻϳ��������Ļ�����壬NH3�ĺ�����С�������������Ĺ�����Ч�ʶ��ܵ�
B�����ڰ���Һ�����������N2��H2��ʵ��������ѭ��ʹ�ã�����������˵���IJ��ʺܸ�
C���ϳɰ���ҵ�ķ�Ӧ�¶ȿ�����500 �棬Ŀ����ʹ��ѧƽ��������Ӧ�����ƶ�
D���ϳɰ������õ�ѹǿ��20 MPa��50 MPa�����ڸ�ѹǿ������ý�Ļ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʣ�
��NaCl���� ������ ��Ba(OH)2��Һ ��ͭ �ݶ�����̼���� ��������ع���
���Ҵ�(C2H5OH) ��Һ̬���� ������̬BaSO4 ��Һ̬SO3
��ش���������(�����)��
�������������ڻ���������� ������ţ���ͬ���������ε����� �� ��������������� , �ܵ������_______________________�����ڵ���ʵ���______________________�����ڷǵ���ʵ���___________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com