
��11�֣�������ijѧϰС����Ҷ����ijЩ���ʽ����о���ѧϰ�Ĺ��̣�
[�о�����]̽���Ҷ����ijЩ����
[��������]�Ҷ��ᣨHOOC��COOH���׳Ʋ��ᣬ����Ҫ�����������£�
| ���� | �Ҷ��� | �Ҷ��ᾧ�� |
| ����ʽ | H2C2O4 | H2C2O4��2H2O |
| ��ɫ״̬ | ��ɫ���� | ��ɫ���� |
| �ܽ�ȣ�g�� | 8.6��20�棩 | �� |
| �۵㣨�棩 | 189.5 | 101.5 |
| �ܶȣ�g��cm��3�� | 1.900 | 1.650 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
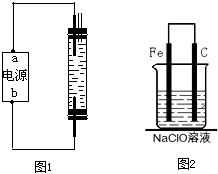 ��2010?��̨һģ��2008��5��12���ҹ��Ĵ��봨�����ش����Ϊ��ֹ�ڴ���֮���߲����У�ȫ�����������������˴����ĸ�������Һ����NaClO��Һ��ijУ̽����ѧϰС�������Һ�������ƣ�NaClO�����Ʊ������ʵȽ�����̽����
��2010?��̨һģ��2008��5��12���ҹ��Ĵ��봨�����ش����Ϊ��ֹ�ڴ���֮���߲����У�ȫ�����������������˴����ĸ�������Һ����NaClO��Һ��ijУ̽����ѧϰС�������Һ�������ƣ�NaClO�����Ʊ������ʵȽ�����̽�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| 7cV |
| 125a |
| 7cV |
| 125a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �Ҷ��� | �Ҷ��ᾧ�� |
| ����ʽ | H2C2O4 | H2C2O4?2H2O |
| ��ɫ״̬ | ��ɫ���� | ��ɫ���� |
| �ܽ�ȣ�g�� | 8.6��20�棩 | - |
| �۵㣨�棩 | 189.5 | 101.5 |
| �ܶȣ�g?cm-3�� | 1.900 | 1.650 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡܼ����У������ѧ�ڵڶ��ο��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��11�֣�������ijѧϰС����Ҷ����ijЩ���ʽ����о���ѧϰ�Ĺ��̣�
[�о�����]̽���Ҷ����ijЩ����
[��������]�Ҷ��ᣨHOOC��COOH���׳Ʋ��ᣬ����Ҫ�����������£�
|
���� |
�Ҷ��� |
�Ҷ��ᾧ�� |
|
����ʽ |
H2C2O4 |
H2C2O4��2H2O |
|
��ɫ״̬ |
��ɫ���� |
��ɫ���� |
|
�ܽ�ȣ�g�� |
8.6��20�棩 |
�� |
|
�۵㣨�棩 |
189.5 |
101.5 |
|
�ܶȣ�g��cm��3�� |
1.900 |
1.650 |
��֪��
������100��ʱ��ʼ������157��ʱ��������������ʼ�ֽ⡣
����Ʋ�����ˮ��
����������ʹ����ʯ��ˮ����ǡ�
���������ڵ����¿�����Ϊ���塣
�������������ṩ����Ϣ���ش��������⣺
[�������]
������һ�����ݲ��ᾧ�����ɶ���ֽ������в���
��Ʒ�����
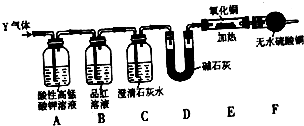
��1����С��ͬѧ���������ΪCO��CO2��H2O����������װ�����һ��̽��ʵ��װ�ã����ᾧ��ֽ�װ���ԣ�װ�ÿ��ظ�ʹ�ã����ӵ�����ȥ����

A��ˮ��װ��ˮ����B��װ����ͭ��C��װ��ˮ����ͭ��D��װ����ʯ��ˮ��E��װ��ʯ��
��ش��������⣺
�� װ�õ�����˳��Ϊ��A��_____________________________________________��
�� ���������CO��ʵ��������____________________________________________________________
�� ����װ���Ƿ���ڲ�����֮���� �����ǻ�����и���ν��___________________________________________________________________________
����������Ҷ������������
��Ʒ�����
��2����С��ͬѧΪ��֤����������������������ʵ�飬�����ܴﵽʵ��Ŀ����______������ĸ����
A�������ᾧ�����ں���̪��NaOH��Һ�У���Һ��ɫ
B���ⶨ��ͬŨ�ȵIJ����������Һ��pH
C���ⶨ�����ƣ�Na2C2O4����Һ��pH
D����������Һ����Na2CO3��Һ�У���CO2�ų�
�����������Ҷ�����л�ԭ��
��Ʒ�����
��3����С��ͬѧ���������ữ��KMnO4��Һ�е�������IJ�����Һ����������KMnO4��Һ��ɫ���Ӷ��жϲ�����н�ǿ�Ļ�ԭ�ԡ���ƽ�÷�Ӧ�����ӷ���ʽ��
___MnO4��+___H2C2O4 +___H+ ===___Mn2+ +___CO2��+___H2O
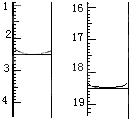
��4����������ԭ���ɶ����ⶨij���ᾧ����Ʒ������H2C2O4��2H2O������һЩ���ʣ���H2C2O4��2H2O�ĺ�����
�����ǣ���ȡ����Ʒ0.12 g��������ˮ��ȫ�ܽ⣬Ȼ����0.020 mol��L��1
������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ�����ζ�ǰ��ζ����е�Һ�������ͼ��ʾ����λ��mL������ò��ᾧ����Ʒ��H2C2O4��2H2O����������Ϊ_____________��
����֪���ԭ��������Mr(H2C2O4��2H2O) =126��
=126��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com