
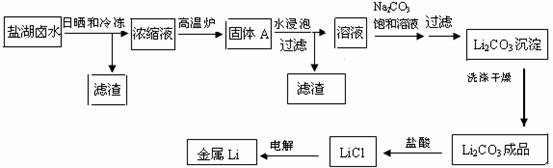
����8�֣����ԭ���ܹ�ҵ�о���ʮ�ֶ��صĵ�λ������Ϊ�����ܽ����������ƶ�����Դ��ҵ�������ǵ�ؼ����ķ�չ���κ�±ˮ��﮵ĺ����ϸߣ���Դ�ḻ������﮵ļ۸��������Ϊ�����������﮵����������κ�±ˮ��Ȼ��ɹ���䶳����±ˮ������ʳ�Ρ�к���Ρ�����þ����±ʯ��һϵ�����࣬�õ�����MgC12��Ũ��ĸҺ������LiC1��Ũ�ȴ�10�����ϡ���Ũ��ĸҺ������¯�ֽ��ȥMgC12��һϵ�й��̣����յõ�����ﮡ�����Ҫ���������������£�
 ��֪��Li2CO3�ڲ�ͬ�¶ȵ��ܽ�����±���
��֪��Li2CO3�ڲ�ͬ�¶ȵ��ܽ�����±���
| �¶�/�� | 0 | 10 | 20 | 50 | 75 | 100 |
| Li2CO3���ܽ��/g | 1.539 | 1.406 | 1.329 | 1.181 | 0.866 | 0.728 |
(1)д��Ũ��ĸҺ�ڸ���¯�зֽ�Ļ�ѧ����ʽ
(2)д��������Li2CO3��Ӧ�����ӷ���ʽ
(3)ϴ������Li2CO3����Ҫ����ˮ������ˮ��ԭ���� ��
(4)����LiCl���Ļ�����
a��������b��������c��������d�������e��He��
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com