
��ʵ�����н��кϳɰ���ҵ��ģ��ʵ�飬����Ӧ����һ��ʱ�䲢����ƽ���Ժ�ʱ�����������������ʼԭ�ϱ���ͬ�ĵ�������������ʵ���������������̣�һ����������1L���ܱ������з������·�Ӧ��
N2��g��+3H2��g�� 2NH3��g������H��0
2NH3��g������H��0
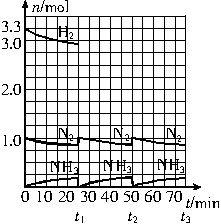
�����ͼʾ�ش��������⣺
��1����Ӧ��ʼʱ�����������������ʵ���֮��n��N2����n��H2����______����15minʱ�ϳɰ���Ӧ��һ�δﵽƽ�⣬�����ʱ����H2��Ũ�ȱ仯��ʾ��ƽ����ѧ��Ӧ����Ϊ________��
��2����t1ʱ�����߷����仯��ԭ����_________________��________________�������������t1���ʱ�����꣨_______��______���Լ���t2ƽ��ʱ�����꣨____��____����
��3��Ϊ�ﵽͼʾ��t2ʱ��ƽ��״̬����t1��t2֮����Ҫ��ȡ�Ĵ�ʩ��___________________��
A.�����������ݻ� B.�����¶� C.�����¶� D.��С�������ݻ�
��4������ʮ��������ѭ�����̣�������t11ƽ��ʱ��N2��H2�����ʵ���֮��n��N2����n��H2����_____________������������N2��H2����ת����֮�Ȧ���N2���æ���H2����_________��
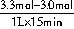 ��0.02mol?L��1?min��1��
��0.02mol?L��1?min��1�� 2NH3��g����
2NH3��g����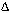 H<0����֪ͨ�����»������������ݻ�����ѹ��ƽ�⼴�����淴Ӧ�����ƶ���
H<0����֪ͨ�����»������������ݻ�����ѹ��ƽ�⼴�����淴Ӧ�����ƶ��� 2NH3��g�����ɵ�����N2������Ϊ1.1mol������H2������Ϊ3.3mol����t11ƽ��ʱ��ʣ��H2Ϊ��6.6mol��3.3mol����3.3mol��ʣ��N2Ϊ��2mol��1.1mol����0.9mol����n��N2����n��H2����0.9mol��3.3mol��3��11������������H2��N2��ת���ʷֱ�Ϊ����H2��=
2NH3��g�����ɵ�����N2������Ϊ1.1mol������H2������Ϊ3.3mol����t11ƽ��ʱ��ʣ��H2Ϊ��6.6mol��3.3mol����3.3mol��ʣ��N2Ϊ��2mol��1.1mol����0.9mol����n��N2����n��H2����0.9mol��3.3mol��3��11������������H2��N2��ת���ʷֱ�Ϊ����H2��= ��100%������N2��=
��100%������N2��=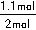 ��100%�������N2�����H2��=11��10��
��100%�������N2�����H2��=11��10�� 


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α�߶���(ѡ��4)��2009��2010ѧ�ꡡ��6�ڡ��ܵ�162�� �˽̿α��(ѡ��4) ���ͣ�058
��ʵ�����н��кϳɰ���ҵ��ģ��ʵ�飬����Ӧ����һ��ʱ�䣬��������Ӧ��ƽ���Ժ�ʱ�����������������ʼԭ�ϱ���ͬ�ĵ�������������ʵ���������������̣���
1 L���ܷ������У�һ�������»ᷢ�����·�Ӧ��N2(g)��3H2(g)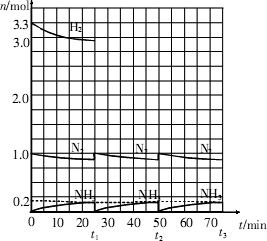
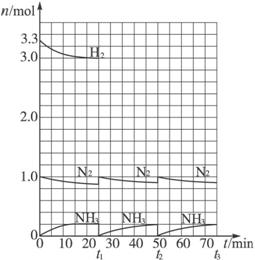
�������ͼ��ʾ�ش��������⣺
(1)��t1ʱ�����߷����仯��ԭ����________�������������t1���ʱ������(________��________)�Լ���t2ƽ��ʱ������(________��________)��
(2)����ʮ������ѭ�����̺���t11�ﵽƽ��ʱ��N2��H2�����ʵ���֮��Ϊn(N2)��n(H2)��________������������N2��H2����ת����֮�Ȧ�(N2)�æ�(H2)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
N2��g��+3H2(g) ![]() 2NH3(g);��H��0
2NH3(g);��H��0

�����ͼʾ�ش��������⣺
��1����Ӧ��ʼʱ�����������������ʵ���֮��n(N2)��n(H2)= _________��_________����15����ʱ�ϳɰ���Ӧ��һ�δﵽƽ�⣬�����H2��Ũ�ȱ仯����ʾ��ƽ����ѧ��Ӧ����Ϊ_________________________��
��2����t1ʱ�����߷����仯��ԭ����__________________��__________________�������������t1���ʱ�����꣨_________��_________���Լ���t2ƽ��ʱ�����꣨_________��_________��������ͼ�л�����t1��t2֮�����������ʵ����仯���ߡ�
��3��Ϊ�ﵽͼʾ��t2��ƽ��״̬����t1��t2֮����Ҫ��ȡ�Ĵ�ʩ��_________��
a.�������������
b.�����¶�
c.�����¶�
d.�����������
��4������ʮ��������ѭ�����̣�������t11�ﵽƽ��ʱ��N2��H2�����ʵ���֮��n(N2)��n(H2)= _________��_________,����������N2��H2����ת����֮�Ȧ�(N2)�æ�(H2)= _________��_________��
��5���������ϼ����������㽨��ϳɰ���Ӧ�����ԭ�ϱ��ǣ�n(N2)��n(H2)=_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ͼʾ�ش��������⣺
(1)��Ӧ��ʼʱ�����������������ʵ���֮��n(N2)��n(H2)��___________����25 minʱ�ϳɰ���Ӧ��һ�δﵽƽ�⣬�����H2��Ũ�ȱ仯����ʾ��ƽ����ѧ��Ӧ����Ϊ_________��
(2)��t1ʱ�����߷����仯��ԭ����_______________��_______________������������ڱ仯��t1���ʱ������(___________��___________)�Լ���t2ƽ��ʱ������(___________��___________)��
(3)����ʮ��������ѭ�����̣�������t11�ﵽƽ��ʱ��N2��H2�����ʵ���֮��n(N2)��n(H2)��___________������������N2��H2����ת����֮�Ȧ�(N2)�æ�(H2)��___________��
(4)�������ϼ����������㽨��ϳɰ���Ӧ�����ԭ�ϱ��ǣ�n(N2)��n(H2)��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
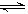 2NH3��g������H��0�������ͼʾ�ش��������⣺
2NH3��g������H��0�������ͼʾ�ش��������⣺
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com