
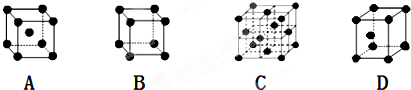
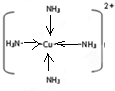
 �ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ
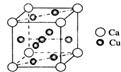
�ǽ���Ca��Cu���γɵ�ij�ֺϽ�ľ����ṹʾ��ͼ����úϽ���Ca��Cu��ԭ�Ӹ�����Ϊ ��ͭ��ij��������ľ����ṹʾ��ͼ���þ�����ܶ�Ϊa g/cm3���谢��٤��������ֵΪNA����þ��������Ϊ
��ͭ��ij��������ľ����ṹʾ��ͼ���þ�����ܶ�Ϊa g/cm3���谢��٤��������ֵΪNA����þ��������Ϊ| m |
| �� |
| 1 |
| 8 |
| 1 |
| 2 |
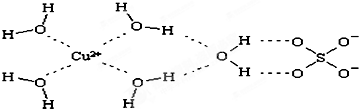
 ���ʴ�Ϊ����ˮ��
���ʴ�Ϊ����ˮ�� ��
��| 1 |
| 8 |
| 1 |
| 2 |
| m |
| �� |
| ||
| a |
| 320 |
| aNA |
| 320 |
| aNA |


 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д� �Ͻ�ƽ��У����ϵ�д�
�Ͻ�ƽ��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2 | B��2.3 |
| C��2.6 | D��2.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
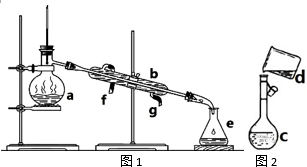 �����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã�
�����������ơ���װ��ʹ�÷�������ѧ��ѧʵ��Ļ�������ͼΪ����ʵ��װ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
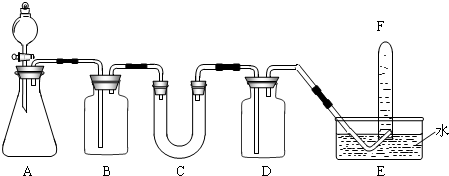
| ���� | �����Լ� | ������Լ���Ŀ�� |
| B | ����NaHCO3��Һ | ���ӷ�������HCl |
| C | ||
| D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(Br-) |
| c(Cl-) |
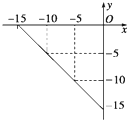
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4��5 | B��1��10 |
| C��10��1 | D��1��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com