
��10�֣�ij����С��ͬѧ����Һ̬�̺�������ʵ��������£�
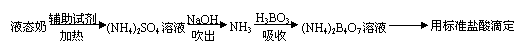
���裺�� ���ձ��м���10.00mLҺ̬�̺����Լ������ȳ�ַ�Ӧ��
�� ����ӦҺת�Ƶ����Թ��У�
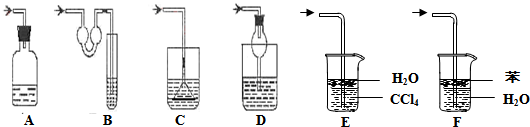
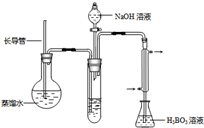
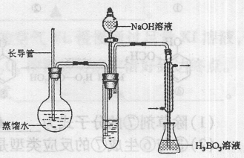
�� ������װ����ˮ������NH3����������H3BO3��Һ���գ�����װ��δ��������
 |
�� ȡ����ƿ���μ�ָʾ������0.1000 mol?L��1�����Һ�ζ���
�� �ظ��ⶨ���Σ�����10.00mL����ˮ����Һ̬�̽�������������
���ݼ�¼���£�
ʵ���� | ��Ʒ�����Լ� | �������������mL�� |
1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50 |
�ش��������⣺
��1���ζ�ʱ(NH4)2B4O7����ת��ΪH3BO3����Ӧ�Ļ�ѧ����ʽΪ_____________________��
��2������۵�ʵ��װ������Ҫ���ȵ�������_____________�����������ƣ��������ܵ�������_________________________________________________��
��3�����4�ſհ���ʵ���Ŀ����____________________________________________��
��4������10.00mLҺ̬���еĺ�����Ӧ����������������� mL����Һ̬�̵ĺ�����Ϊ____________mg?mL��1��
 �ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
�ƸԺ���ȫ�����Ų��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| �����Լ� |
| ���� |
| NaOH��Һ |
| ���� |
| H3BO3 |
| ���� |

| ʵ���� | ��Ʒ�����Լ� | �����������/mL |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 25.50 |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
| 4 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
| 5 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 1.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��ȡ����ƿ���μ�ָʾ������0.1000mol?L-1�����Һ�ζ���
��ȡ����ƿ���μ�ָʾ������0.1000mol?L-1�����Һ�ζ���| ʵ���� | ��Ʒ�����Լ� | �������������mL�� |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
| 4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ���� |
| ||
| ���� |
| ||
| ���� |

| ʵ���� | ��Ʒ�����Լ� | �������� �����mL�� |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
| 4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�����и�����ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��12�֣�������A��B��С�⣬�ֱ��Ӧ�ڡ����ʽṹ�����ʡ��͡�ʵ�黯ѧ������ѡ��ģ������ݡ���ѡ������һ�⣬������Ӧ�Ĵ������������������ⶼ������A�����֡�
A����1��ƫ���ᱵ�����ȶ��Ժã���糣���ߣ���С�ͱ�ѹ������Ͳ���������ж���Ӧ�á��Իش��������⣺
��TiԪ����Ԫ�����ڱ��е�λ���� �����̬ԭ�ӵ�
�����Ų�ʽΪ ��
��ƫ���ᱵ�����о����Ľṹʾ��ͼ����ͼ�����Ļ�ѧʽ�� ����������ÿ����Ti�����ڵ���ԭ����Ϊ ����

��2������[��CN��2]��ɫ���綾���п�����ζ����±�ص����������ơ���֪������м����֮��ļн�Ϊ180�������жԳ��ԣ���ṹʽΪ ����CNһ��Ϊ�ȵ�����ĵ��ʵķ���ʽΪ ��
B��ij����С��ͬѧ����Һ̬�̺�������ʵ��������£�

���裺
�����ձ��м���10��00mLҺ̬�̺����Լ�������
��ַ�Ӧ��
�ڽ���ӦҺת�Ƶ����Թ��У�
�۰���ͼװ����ˮ������NH3����������H3BO3��Һ���գ�����װ��δ��������
��ȡ����ƿ���μ�ָʾ������0��1000mol��L-1����
��Һ�ζ���
���ظ��ⶨ���Σ�����10��00mL����ˮ����Һ̬�̽�
������������
���ݼ�¼���£�

�ش��������⣺
��1���ζ�ʱ��NH4��2B4O7����ת��ΪH3BO3����Ӧ�����ӷ���ʽΪ ��
��2������۵�ʵ��װ������Ҫ���ȵ������� �����������ƣ��������ܵ������� ��
��3�������հ���ʵ���ʵ�����к�Ӱ�� �����Ӱ�족����ƫ�ߡ�����ƫ�͡�����
��4������10��00mLҺ̬���еĺ�����Ӧ���˼������������� mL����Һ̬�̵ĺ����� mg��mL-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011���㽭ʡ������Ϫ�к�����ѧ�߿���ѧģ���Ծ��������棩 ���ͣ������

| ʵ���� | ��Ʒ�����Լ� | �������������mL�� |
| 1 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.45 |
| 2 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.55 |
| 3 | 10.00mLҺ̬�̡�0.2g������20mLŨ���� | 33.50 |
| 4 | 10.00mL����ˮ��0.2g������20mLŨ���� | 1.50 |


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com