
ĻĀĮŠĶĘĀŪÕżČ·µÄŹĒ(””””)
A£®S(g)£«O2(g)===SO2(g)””¦¤H1£»S(s)£«O2===SO2(g)””¦¤H2£¬Ōņ¦¤H1£¾¦¤H2
B£®C(ŹÆÄ«£¬s)===C(½šøÕŹÆ£¬s)””¦¤H£½£«1.9 kJ·mol£1£¬ŌņÓÉŹÆÄ«ÖĘČ”½šøÕŹÆµÄ·“Ó¦ŹĒĪüČČ·“Ó¦£¬½šøÕŹÆ±ČŹÆÄ«ĪȶØ
C£®NaOH(aq)£«HCl(aq)===NaCl(aq)£«H2O(l)””¦¤H£½£57.4 kJ·mol£1£¬Ōņ£ŗŗ¬20 g NaOHµÄĻ”ČÜŅŗÓėĻ”ŃĪĖįĶźČ«·“Ó¦£¬·Å³öµÄČČĮæĪŖ28.7 kJ
D£®2C(s)£«O2(g)===2CO(g)””¦¤H£½£221 kJ·mol£1£¬ŌņĢ¼µÄČ¼ÉÕČȵČÓŚ110.5 kJ·mol£1

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼ĖłŹ¾µÄøÖĢśøÆŹ“ÖŠ£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
 ””
””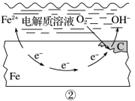
A£®Ģ¼±ķĆę·¢ÉśŃõ»Æ·“Ó¦
B£®øÖĢś±»øÆŹ“µÄ×īÖÕ²śĪļĪŖFeO
C£®Éś»īÖŠøÖĢśÖĘĘ·µÄøÆŹ“ŅŌĶ¼¢ŚĖłŹ¾ĪŖÖ÷
D£®Ķ¼¢ŚÖŠ£¬Õż¼«·“Ó¦Ź½ĪŖO2£«4e££«2H2O===4OH£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌõŃłÓĆ»·ŠĪ²£Į§½Į°č°ō½Į°čČÜŅŗ£¬²»ÄÜÓĆĶĖæ½Į°č°ō“śĢęµÄĄķÓÉŹĒŹ²Ć“£æ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĒāĘųŹĒŅ»ÖÖĒå½ąÄÜŌ“£¬ĒāĘųµÄÖĘČ”Óė“¢“ęŹĒĒāÄÜŌ“ĄūÓĆĮģÓņµÄŃŠ¾æČČµć”£
ŅŃÖŖ£ŗCH4(g)£«H2O(g)===CO(g)£«3H2(g)
¦¤H£½206.2 kJ·mol£1
CH4(g)£«CO2(g)===2CO(g)£«2H2(g)
¦¤H£½247.4 kJ·mol£1
2H2S(g)===2H2(g)£«S2(g)
¦¤H£½169.8 kJ·mol£1
(1)ŅŌ¼×ĶéĪŖŌĮĻÖĘČ”ĒāĘųŹĒ¹¤ŅµÉĻ³£ÓƵÄÖĘĒā·½·Ø”£
CH4(g)ÓėH2O(g)·“Ӧɜ³ÉCO2(g)ŗĶH2(g)µÄČČ»Æѧ·½³ĢŹ½ĪŖ________________________________________________________________________”£
(2)H2SČČ·Ö½āÖĘĒāŹ±£¬³£Ļņ·“Ó¦Ę÷ÖŠĶØČėŅ»¶Ø±ČĄżæÕĘų£¬Ź¹²æ·ÖH2SČ¼ÉÕ£¬ĘäÄæµÄŹĒ____________________________£»Č¼ÉÕÉś³ÉµÄSO2ÓėH2S½ųŅ»²½·“Ó¦£¬Éś³ÉĪļŌŚ³£ĪĀĻĀ¾ł·ĒĘųĢ壬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ_____________________________________________”£
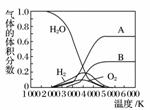
(3)H2OµÄČČ·Ö½āŅ²æɵƵ½H2£¬øßĪĀĻĀĖ®·Ö½āĢåĻµÖŠÖ÷ŅŖĘųĢåµÄĢå»ż·ÖŹżÓėĪĀ¶ČµÄ¹ŲĻµČēĻĀĶ¼ĖłŹ¾”£Ķ¼ÖŠA”¢B±ķŹ¾µÄĪļÖŹŅĄ“ĪŹĒ________________________”£
(5)Mg2CuŹĒŅ»ÖÖ“¢ĒāŗĻ½š”£350 ”ꏱ£¬Mg2CuÓėH2·“Ó¦£¬Éś³ÉMgCu2ŗĶ½öŗ¬Ņ»ÖÖ½šŹōŌŖĖŲµÄĒā»ÆĪļ(ĘäÖŠĒāµÄÖŹĮæ·ÖŹżĪŖ0.077)”£Mg2CuÓėH2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________________________________________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŅŅ“¼ŹĒÖŲŅŖµÄÓŠ»ś»Æ¹¤ŌĮĻ£¬æÉÓÉŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·Ø»ņ¼ä½ÓĖ®ŗĻ·ØÉś²ś”£»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)¼ä½ÓĖ®ŗĻ·ØŹĒÖøĻČ½«ŅŅĻ©ÓėÅØĮņĖį·“Ӧɜ³ÉĮņĖįĒāŅŅõ„(C2H5OSO3H)£¬ŌŁĖ®½āÉś³ÉŅŅ“¼”£Š“³öĻąÓ¦·“Ó¦µÄ»Æѧ·½³ĢŹ½_____________________________________________
________________________________________________________________________ӣ
(2)ŅŃÖŖ£ŗ
¼×“¼ĶŃĖ®·“Ó¦2CH3OH(g)===CH3OCH3(g)£«H2O(g)
¦¤H1£½£23.9 kJ·mol£1
¼×“¼ÖĘĻ©Ģž·“Ó¦2CH3OH(g)===C2H4(g)£«2H2O(g)
¦¤H2£½£29.1 kJ·mol£1
ŅŅ“¼Ņģ¹¹»Æ·“Ó¦C2H5OH(g)===CH3OCH3(g)
¦¤H3£½£«50.7 kJ·mol£1
ŌņŅŅĻ©ĘųĻąÖ±½ÓĖ®ŗĻ·“Ó¦C2H4(g)£«H2O(g)===C2H5OH(g)µÄ¦¤H£½______________ kJ·mol£1”£
Óė¼ä½ÓĖ®ŗĻ·ØĻą±Č£¬ĘųĻąÖ±½ÓĖ®ŗĻ·ØµÄÓŵćŹĒ_____________________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ(””””)
A£®ČĪŗĪĖįÓė¼ī·¢ÉśÖŠŗĶ·“Ӧɜ³É1 mol H2OµÄ¹ż³ĢÖŠ£¬ÄÜĮæ±ä»Æ¾łĻąĶ¬
B£®Ķ¬ĪĀĶ¬Ń¹ĻĀ£¬H2(g)£«Cl2(g)===2HCl(g)ŌŚ¹āÕÕŗĶµćČ¼Ģõ¼žĻĀµÄ¦¤HĻąĶ¬
C£®ŅŃÖŖ£ŗ¢Ł2H2(g)£«O2(g)===2H2O(g)””¦¤H1£½£a kJ·mol£1£¬¢Ś2H2(g)£«O2(g)===2H2O(l)
¦¤H2£½£b kJ·mol£1£¬Ōņa>b
D£®ŅŃÖŖ£ŗ¢ŁC(ŹÆÄ«£¬s)£«O2(g)===CO2(g)””¦¤H1£½£393.5 kJ·mol£1£¬¢ŚC(½šøÕŹÆ£¬s)£«O2(g)===
CO2(g)””¦¤H2£½£395.0 kJ·mol£1£¬Ōņ½šøÕŹÆ±ČŹÆÄ«ĪȶØ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
25 ”ꏱ£¬ĻąĶ¬ĪļÖŹµÄĮæÅØ¶ČµÄĻĀĮŠČÜŅŗ£ŗ¢ŁNaCl””¢ŚNaOH””¢ŪH2SO4””¢Ü(NH4)2SO4£¬ĘäÖŠĖ®µÄµēĄė³Ģ¶Č°“Óɓ󵽊”Ė³ŠņÅÅĮŠµÄŅ»×éŹĒ(””””)
A£®¢Ü>¢Ū>¢Ś>¢Ł B£®¢Ś>¢Ū>¢Ł>¢Ü
C£®¢Ü>¢Ł>¢Ś>¢Ū D£®¢Ū>¢Ś>¢Ł>¢Ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ä³Ń§ÉśÓĆŅŃÖŖĪļÖŹµÄĮæÅØ¶ČµÄŃĪĖįĄ“²ā¶ØĪ“ÖŖĪļÖŹµÄĮæÅØ¶ČµÄNaOHČÜŅŗŹ±£¬Ń”Ōń¼×»ł³Č×÷ÖøŹ¾¼Į”£ĒėĢīŠ“ĻĀĮŠæÕ°×£ŗ
(1)ÓƱź×¼µÄŃĪĖįµĪ¶Ø“ż²āµÄNaOHČÜŅŗŹ±£¬×óŹÖĪÕĖįŹ½µĪ¶Ø¹ÜµÄ»īČū£¬ÓŅŹÖŅ”¶Æ׶ŠĪĘ棬ŃŪ¾¦×¢ŹÓ________£¬Ö±µ½Ņņ¼ÓČėŅ»µĪŃĪĖįŗó£¬ČÜŅŗÓÉ»ĘÉ«±äĪŖ³ČÉ«£¬²¢______ĪŖÖ¹”£
(2)ĻĀĮŠ²Ł×÷ÖŠæÉÄÜŹ¹Ėł²āNaOHČÜŅŗµÄÅØ¶ČŹżÖµĘ«µĶµÄŹĒ________(Ģī×ÖÄøŠņŗÅ)”£
A£®ĖįŹ½µĪ¶Ø¹ÜĪ“ÓƱź×¼ŃĪĖįČóĻ“¾ĶÖ±½Ó×¢Čė±ź×¼ŃĪĖį
B£®µĪ¶ØĒ°Ź¢·ÅNaOHČÜŅŗµÄ׶ŠĪĘæÓĆÕōĮóĖ®Ļ“¾»ŗóƻӊøÉŌļ
C£®ĖįŹ½µĪ¶Ø¹ÜŌŚµĪ¶ØĒ°ÓŠĘųÅŻ£¬µĪ¶ØŗóĘųÅŻĻūŹ§
D£®¶ĮČ”ŃĪĖįĢå»żŹ±£¬æŖŹ¼ŃöŹÓ¶ĮŹż£¬µĪ¶Ø½įŹųŹ±ø©ŹÓ¶ĮŹż
(3)ČōµĪ¶ØæŖŹ¼ŗĶ½įŹųŹ±£¬ĖįŹ½µĪ¶Ø¹ÜÖŠµÄŅŗĆęČēĶ¼ĖłŹ¾£¬ŌņĘšŹ¼¶ĮŹżĪŖ________mL£¬ÖÕµć¶ĮŹżĪŖ________mL£¬
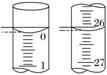
ĖłÓĆŃĪĖįČÜŅŗµÄĢå»żĪŖ________mL”£
(4)Ä³Ń§Éśøł¾Ż3“ĪŹµŃé·Ö±š¼ĒĀ¼ÓŠ¹ŲŹż¾ŻČēĻĀ±ķ£ŗ
| µĪ¶Ø“ĪŹż | “ż²āNaOHČÜŅŗµÄĢå»ż/mL | 0.100 0 mol·L£1ŃĪĖįµÄĢå»ż/mL | ||
| µĪ¶ØĒ°æĢ¶Č | µĪ¶ØŗóæĢ¶Č | ČÜŅŗĢå»ż/mL | ||
| µŚŅ»“Ī | 25.00 | 0.00 | 26.11 | 26.11 |
| µŚ¶ž“Ī | 25.00 | 1.56 | 30.30 | 28.74 |
| µŚČż“Ī | 25.00 | 0.22 | 26.31 | 26.09 |
ŅĄ¾ŻÉĻ±ķŹż¾ŻĮŠŹ½¼ĘĖćøĆNaOHČÜŅŗµÄĪļÖŹµÄĮæÅØ¶Č”£
“š°ø””(1)׶ŠĪĘæÖŠČÜŅŗŃÕÉ«±ä»Æ””ŌŚ°ė·ÖÖÓÄŚ²»±äÉ«
(2)D””(3)0.00””26.10””26.10
(4)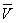 £½
£½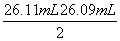 £½26.10 mL£¬c(NaOH)£½
£½26.10 mL£¬c(NaOH)£½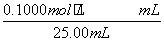 £½0.104 4 mol·L£1
£½0.104 4 mol·L£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Cu£«»łĢ¬ŗĖĶāµē×ÓÅŲ¼Ź½ĪŖ
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com