
| A������Һ�������Ϊ��صĵ���� |
| B�������һ��ˮ��Һ�ʼ��� |
| C�������һ��ˮ������ӷ���ʽ��ʾΪ��C2H5NH3++H2O�TC2H5NH2+H3O+ |
| D����ͬ�¶��£���ͬ���ʵ���Ũ�ȵ������һ����Һ���������Һǰ�ߵ�pHֵС |


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| n(NH3) |
| n(CO2) |
| n(H2O) |
| n(CO2) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2.2mol/L |
| B��4mol/L |
| C��5mol/L |
| D��6.25mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ͨ��CH3OH�ĵ缫Ϊ���� |
| B�����ŷŵ�Ľ��У���������pH���� |
| C��ÿ����1mol CH3OH���������·�ṩ6mol e- |
| D��ͨ��O2��һ���缫��ӦΪ4H2O+2O2-+8e-�T8OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ϼ�Ϊ+3�� |
| B�������γɻ�ѧʽΪKXO3���� |
| C�����⻯�������������Ȫʵ�� |
| D��������������ˮ������ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1L 0.1mol/L��̼������Һ��C��Na+����C��CO32-��=2��1 |
| B��25��ʱNaOH��Һ��ˮ��Kw����100��ʱNaCl��Һ��ˮ��Kw |
| C���к������ͬpH��ȵ�����ʹ�����Һ�����ĵ����ʵ���Ũ�ȵ�NaOH��Һ�������������� |
| D��25��ʱ��pH=8��0.1mol?L-1 NaX��Һ����ˮ�������c��OH-��=10-6mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����״���£�2.24L������̼�к��й��õ��ӶԵ���ĿΪ0.2NA |
| B��25��ʱ��pH=12��Na2CO3��Һ�к���OH-����ĿΪ0.01NA |
| C�����³�ѹ�£�28g��ϩ�ͱ�ϩ�Ļ�������к���̼ԭ�ӵ���ĿΪ2NA |
| D��0.1mol Cl2���������۷�Ӧת�Ƶ��ӵ���ĿΪ0.3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
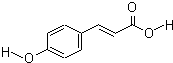 ���ǻ��������һ��ǿЧ�ĵ�����ϣ���Һ����ʾ����ҵ�н������о��㷺���ṹ��ʽ��ͼ��
���ǻ��������һ��ǿЧ�ĵ�����ϣ���Һ����ʾ����ҵ�н������о��㷺���ṹ��ʽ��ͼ���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com