
 �����������Һ��Ũ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�����������Һ��Ũ����ʲôӰ�죿���ƫ�ߡ�����ƫ�͡�����Ӱ�족��
 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
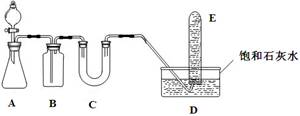
|
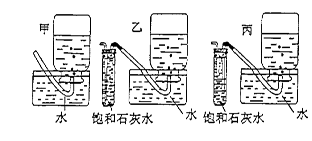
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ��������ƽ������ʱ������ʹ�õ���������Ʒ��
��������ƽ������ʱ������ʹ�õ���������Ʒ��  ������ţ�����ȱ�ٵ������� ��
������ţ�����ȱ�ٵ������� �� �ߵ��� ������ţ�
�ߵ��� ������ţ� ʱ���ӿ̶�
ʱ���ӿ̶��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע������� |
| B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��� |
�鿴�𰸺ͽ���>>
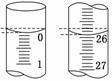
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
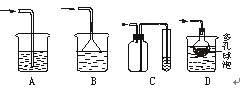
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
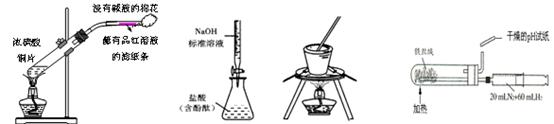
| A����ȡSO2������SO2��Ư���� | B���ⶨ����Ũ�ȼ��� |
| C����ʳ��ˮ����ȡNaCl | D���ϳɰ����������� |
�鿴�𰸺ͽ���>>
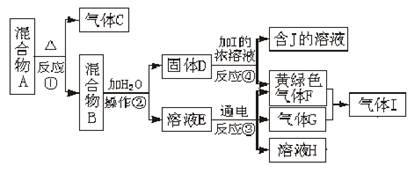
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ������Ũ��ʹNaCl���������������������������˳���ź������ǡ���
������Ũ��ʹNaCl���������������������������˳���ź������ǡ��� | A���٢ڢۢܢݢޡ��� | B���ۢ٢ݢܢڢޡ��� | C���ڢ٢ۢܢݢ� | D���ڢۢݢܢ٢� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��4�� | B��5�� | C��6�� | D��7�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com