
���� ��1����Ԫ�ػ��ϼ�+2�۱仯Ϊ+3�ۣ���Ԫ�ػ��ϼ�-1�۱仯Ϊ+6�ۣ�FeS2������Ӧ����ת��15�����ӣ�HNO3�е�Ԫ�ػ��ϼ�+5�۱仯Ϊ+2�ۣ�����ת��3�������������ʽǰϵ��Ϊ5������ԭ���غ���ƽ�õ���ѧ����ʽΪ
��2��ʵ����������ˮʱ�������ձ��м���3�����Ũ���ᣬȻ���ټ���1�����Ũ���ᣬ�ӱ߽��裬��Ͼ��ȣ�
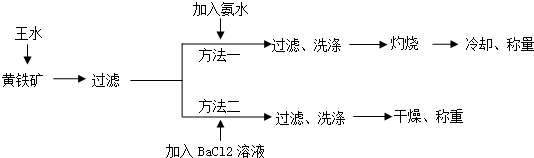
��3������һ�й��ˡ�ϴ���ò����������������ò��������裻
��4����������Ҫ�ж�BaCl2��Һ�Ƿ������������Һ�м�������BaCl2���������������ɫ��������BaCl2��Һ���㣬���������Ҳ��������Һ�м���Na2SO4���������BaSO4��ɫ������֤��BaCl2��Һ�����������㣻
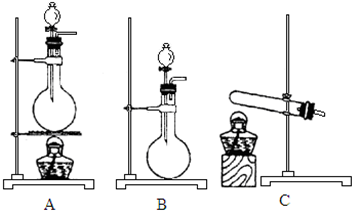
��5����ѡ���Լ���Ũ����͢�ʳ�ι�����ȡHCl����Ҫ���ȣ�Ӧѡ��װ��A��������Ӧ�Ļ�ѧ����ʽΪ2NaCl+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Na2SO4+2HCl�����NaCl+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$NaHSO4+HCl������ѡ���Լ���Ũ����͢�Ũ������ȡHCl������Ũ������ˮ�����ȣ�����Ũ����ӷ�������Ҫ���ȣ�ѡ��װ��B��
��6������һ�У�����ʱ���������ֽ�������������������Ӧ�Ļ�ѧ��Ӧ����ʽΪ2Fe��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Fe2O3+3H2O��
���ݹ�ϵʽ��2FeS2��Fe2O3��
240 160
m��FeS2�� 0.8g��
m��FeS2��=$\frac{240��0.8g}{160}$=1.2g����ÿ�ʯ��FeS2������������$\frac{1.2g}{1.5g}$��100%=40%��
��� �⣺��1��FeS2+HNO3+HCl��FeCl3+H2SO4+NO��+H2O����Ԫ�ػ��ϼ�+2�۱仯Ϊ+3�ۣ���Ԫ�ػ��ϼ�-1�۱仯Ϊ+6�ۣ�FeS2������Ӧ����ת��15�����ӣ�HNO3�е�Ԫ�ػ��ϼ�+5�۱仯Ϊ+2�ۣ�����ת��3�������������ʽǰϵ��Ϊ5������ԭ���غ���ƽ�õ���ѧ����ʽΪ��FeS2+5HNO3+3HCl$\frac{\underline{\;\;��\;\;}}{\;}$FeCl3+2H2SO4+5NO��+2H2O��
�ʴ�Ϊ��FeS2+5HNO3+3HCl$\frac{\underline{\;\;��\;\;}}{\;}$FeCl3+2H2SO4+5NO��+2H2O��
��2��ʵ����������ˮʱ�������ձ��мӣ���3�����Ũ���ᣬȻ���ټ���1�����Ũ���ᣬ�ӱ߽��裬��Ͼ��ȣ�
�ʴ�Ϊ������Ͳȡ3�����Ũ���ᵹ���ձ��У���ȡ1�����Ũ������������Ũ�����У��ӱ߽��裻
��3������һ�й��ˡ�ϴ���ò����������������ò��������裬�ʴ�Ϊ����������
��4����������Ҫ�ж�BaCl2��Һ�Ƿ������������Һ�м�������BaCl2���������������ɫ��������BaCl2��Һ���㣬���������Ҳ��������Һ�м���Na2SO4���������BaSO4��ɫ������֤��BaCl2��Һ�����������㣻
�ʴ�Ϊ��BD��
��5����ѡ���Լ���Ũ����͢�Ũ������ȡHCl������Ũ������ˮ�����ȣ�����Ũ����ӷ�������Ҫ���ȣ�ѡ��װ��B��
��ѡ���Լ���Ũ����͢�ʳ�ι�����ȡHCl����Ҫ���ȣ�Ӧѡ��װ��A��������Ӧ�Ļ�ѧ����ʽΪ2NaCl+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Na2SO4+2HCl�����NaCl+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$NaHSO4+HCl����
�ʴ�Ϊ��B��A��2NaCl+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$Na2SO4+2HCl����NaCl+H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$NaHSO4+HCl����
��6������һ�У�����ʱ���������ֽ�������������������Ӧ�Ļ�ѧ��Ӧ����ʽΪ2Fe��OH��3$\frac{\underline{\;\;��\;\;}}{\;}$Fe2O3+3H2O��
���ݹ�ϵʽ��2FeS2��Fe2O3��
240 160
m��FeS2�� 0.8g��
m��FeS2��=$\frac{240��0.8g}{160}$=1.2g��
��ÿ�ʯ��FeS2������������$\frac{1.2g}{1.5g}$��100%=40%��
�ʴ�Ϊ��40%��
���� ���⿼���������FeS2�����IJⶨ����ȷʵ����������ǽ���Ĺؼ������շ��������ͻ����ǹؼ�����Ŀ�Ѷ��еȣ�


 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢܢޢ� | B�� | �٢ܢݢ� | C�� | �٢ܢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��CnH2n+2 | B�� | ��CnH2n+2O | C�� | ȩCnH2nO | D�� | ����CnH2nO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ������������������ֱ���ȫȼ�գ����߷ų��������� | |
| B�� | �ɵ���Aת��Ϊ����B��H=+119kJ•mol-1����֪����B�ȵ���A�ȶ� | |
| C�� | ϡ��Һ�У�H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ•mol-1 | |
| D�� | ��25��C��101kPaʱ��2g H2��ȫȼ������Һ̬ˮ���ų�285.8kJ�������ʾH2ȼ���ȵĻ�ѧ����ʽ��2H2��g��+O2��g���T2H2O��l����H=-571kJ•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������Һ��ͨ������CO2��2C6H5O-+CO2+H2O��2C6H5OH+CO32- | |
| B�� | FeSO4��������Һ�м�H2O2��2Fe2++H2O2+2H+�T2Fe3++2H2O | |
| C�� | �ð�ˮ����������SO2��NH3•H2O+SO2�TNH4++HSO3- | |
| D�� | ̼��������Һ�м������ʯ��ˮ��HCO3-+OH-�TCO32-+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
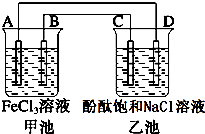 ��ͼ��ʾ���׳��е�ط�ӦʽΪ2Fe3++Cu�T2Fe2++Cu2+����Ӧ������A�缫������С��C��DΪʯī�缫���ҳ���Ϊ200mL�μӷ�̪�ı���NaCl��Һ���ش��������⣺
��ͼ��ʾ���׳��е�ط�ӦʽΪ2Fe3++Cu�T2Fe2++Cu2+����Ӧ������A�缫������С��C��DΪʯī�缫���ҳ���Ϊ200mL�μӷ�̪�ı���NaCl��Һ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ���� | CH3COONa | NaHCO3 | Na2CO3 | NaClO | NaCN |
| pH | 8.8 | 9.7 | 11.6 | 10.3 | 11.1 |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ҫ����ϩ���Ƿ���������ױ���Ӧ�ȼ�������ˮ��Ȼ���ټ������Ը��������Һ | |
| B�� | ����Ũ�����Ũ����Ļ���ʱ����Ũ�����رڻ������뵽Ũ������ | |
| C�� | ��������ʱ����ʢ�л��Һ���Թ�ֱ���ھƾ��ƻ����ϼ��� | |
| D�� | ��ȥ�屽���������壬���Լ�ˮ���Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com