
| ʵ�� ��� | HA�����ʵ��� Ũ�ȣ�mol•L-1�� | NaOH�����ʵ��� Ũ�ȣ�mol•L-1�� | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
���� ��1�������ʵ������ʱ������ǡ�÷�Ӧ�����Σ�������Һ��pH�ж�����ǿ����
��2���κ���Һ�ж����ڵ���غ㣬���ݵ���غ��жϣ�
��3�������Һ������Ϊ�����ʵ�����HA��NaA��pH��7˵��A-��ˮ�����HA�ĵ��룬��ϵ���غ��жϣ�
��4���ɵ���غ��ϵʽ���ε�c��Na+��-c��A-��=c��OH-��-c��H+����
��5�����ݶ�Ԫ��ĵ��뷽��ʽ֪��B2-ֻ������һ��ˮ�⣬��ϵ���غ�������غ�������
��� �⣺��1����HA��ǿ�ᣬǡ����NaOH��Һ��Ӧ����ǿ��ǿ���Σ�pH=7����HA�����ᣬ���ɵ�NaAˮ���Լ��ԣ�pH��7��
�ʴ�Ϊ��a=7ʱ��HA��ǿ�a��7ʱ��HA�����
��2�������Һ�д��ڵ���غ�c��Na+��+c��H+��=c��A-��+c��OH-��������pH=7����c��Na+��=c��A-����
�ʴ�Ϊ��C��
��3�������Һ������Ϊ�����ʵ�����HA��NaA��pH��7˵��A-��ˮ�����HA�ĵ��룬��������Ũ���ɴ�С��˳��Ϊc��Na+����c��A-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��A-����c��OH-����c��H+����
��4���ɵ���غ��ϵʽ���ε�c��Na+��-c��A-��=c��OH-��-c��H+��=��10-4-10-10��mol•L-1��
�ʴ�Ϊ��10-4-10-10��
��5����Na2B�д���ˮ��ƽ�⣺B2-+H2O=HB-+OH-��HB-�����һ��ˮ�⣬������Һ��û��H2B���ӣ�
A�����������غ��c��B2-��+c��HB-��=0.1mol•L-1����A��ȷ��
B��HB-�����һ��ˮ�⣬������Һ��û��H2B���ӣ���B����
C�����������غ��c��OH-��=c��H+��+c��HB-������C��ȷ��
D�����ݵ���غ��c��Na+��+c��H+��=c��OH-��+c��HB-��+2c��B2-������D����
�ʴ�Ϊ��AC��
���� ���⿼����������ʵĵ��롢����Ũ�ȴ�С�ıȽϣ���ȷ������ʵ����ص��������غ㡢����غ�������غ������������ע�⣨5����HB-��ˮ�⣬Ϊ�״��㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ�е�pHǰ�ߴ� | |
| B�� | ���߽�����ˮ��ƽ�⣬�����ڵ���ƽ�� | |
| C�� | ����Һ�о�����c��Na+��+c��H+��=c��HCO${\;}_{3}^{-}$��+2c��CO${\;}_{3}^{2-}$��+c��OH-�� | |
| D�� | �ֱ����NaOH���壬�ָ���ԭ�¶ȣ�c��CO${\;}_{3}^{2-}$��ǰ���������С |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CCl4�ĵ���ʽ��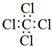 | B�� | ��ϩ�����ģ�� | ||
| C�� | ��������ʽ��CH2O | D�� | ��ϩȩ�Ľṹ��ʽ��CH2CHCHO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | -16QkJ/mol | B�� | -5Q kJ/mol | C�� | -4Q kJ/mol | D�� | -2Q kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | CaO | B�� | NaOH | C�� | CO2 | D�� | Fe3O4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��SO42-��HCO3- | B�� | NH4+��SO42-��NO3- | C�� | Cu2+��ClO-��NO3- | D�� | K+��OH-��Cl- |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com