
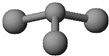
 A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������Bԭ�ӵ��������������������������2����A��һ��ԭ���У���������������֮��Ϊ�㣮DԪ�ص�ԭ������������Ϊm������������Ϊn��EԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊ
A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������Bԭ�ӵ��������������������������2����A��һ��ԭ���У���������������֮��Ϊ�㣮DԪ�ص�ԭ������������Ϊm������������Ϊn��EԪ�ص�ԭ��L�������Ϊm+n��M�������Ϊ| m |
| 2 |
| m |
| 2 |
| 6 |
| 2 |
| m |
| 2 |
| 6 |
| 2 |
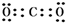 ���ṹʽΪO=C=O��
���ṹʽΪO=C=O��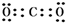 ��O=C=O��
��O=C=O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
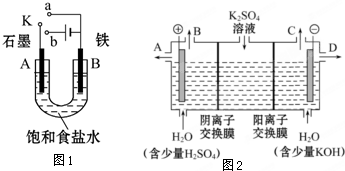 ij����С������ͼ1װ�ý���ʵ�飬�Իش��������⣮
ij����С������ͼ1װ�ý���ʵ�飬�Իش��������⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
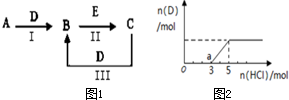 A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ1��ʾ��
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ1��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
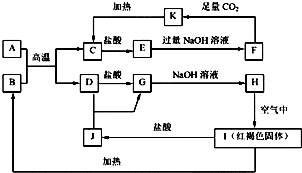
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
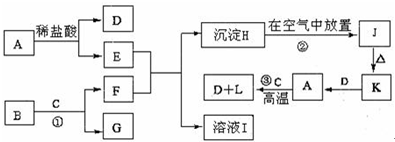
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
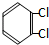 ��
�� 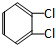
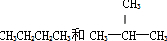
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������pHС��5.6����ˮ |
| B�����ܼ��ŷ��ϵ�̼���õ�Ҫ�� |
| C��PM2.5���ڴ�����Ⱦ�� |
| D��CO2��NO2��SO2���ᵼ��������γ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com