
����Ŀ��ij�����A����KAl��SO4��2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮���ת����
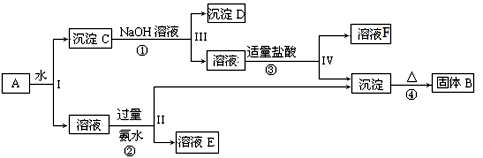
�ݴ˻ش��������⣺
(1)I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ�����______��
(2)������ͼ��Ӧ��ϵ��д������B��F�������ʳɷֵĻ�ѧʽ��B______��F_____��
(3)д�����̷�Ӧ�ٵ����ӷ���ʽ_________________������B������Ӧ�Ľ����䵥��������������ڸ����·�Ӧ�Ļ�ѧ����ʽ__________________________��
(4)д�������������ʱ�����ӷ�Ӧ����ʽ_____________��
���𰸡���10�֣� ��1�����ˣ�2�֣���2��Al2O3��AlCl3����1�֣���3��Al2O3+2OH��=2AlO2��+H2O��
3Fe3O4��8Al![]() 4Al2O3+ 9Fe ����2�֣���4��AlO2��+ 4H+ = Al3+ + 2H2O��2�֣�
4Al2O3+ 9Fe ����2�֣���4��AlO2��+ 4H+ = Al3+ + 2H2O��2�֣�
��������
�����̿�֪��Al2O3��Fe2O3������ˮ�������CΪAl2O3��Fe2O3������������Ӧ�������DΪFe2O3����Ӧ�ڢ������ɵij���ΪAl��OH��3����ҺFΪ�Ȼ��ƣ������������ȷֽ���������������BΪAl2O3����Ӧ��ΪKAl��SO4��2�백ˮ�ķ�Ӧ������ҺEΪK2SO4����NH4��2SO4��NH3��H2O��Ȼ�������ʵ����ʷ������
�������Ϸ�����֪BΪAl2O3��CΪAl2O3��Fe2O3��DΪFe2O3����ҺEΪK2SO4����NH4��2SO4��NH3��H2O����ҺFΪ�Ȼ��ƣ���
��1�����벻���Թ������Һ�ķ���Ϊ���ˣ����Ԣ��IJ��ж�����Һ�ͳ����ķ��뷽��Ϊ���ˣ�
��2��������������֪��BΪAl2O3��FΪNaCl��
��3����Ӧ�������������������Ʒ�Ӧ�������ӷ���ʽΪAl2O3+2OH����2AlO2��+H2O������B������Ӧ�Ľ���������������������������ڸ����·������ȷ�Ӧ�����ɵ�����������������Ӧ����ʽΪ3Fe3O4��8Al![]() 4Al2O3+9Fe��
4Al2O3+9Fe��
��4��ƫ�������������ᷴӦ���������Ӻ�ˮ�����ӷ�Ӧ����ʽΪAlO2��+4H+��Al3++2H2O��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�У�ˮ����ԭ������
A. Mg��2H2O![]() Mg(OH)2��H2��
Mg(OH)2��H2��
B. 2F2+2H2O�T4HF+O2
C. Cl2��H2O![]() HCl+HClO
HCl+HClO
D. 2Na2O2+2H2O�T4NaOH+O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����淴Ӧ��aA��g��+bB��g��![]() cC��g��+dD��g��������ͼ�ش�
cC��g��+dD��g��������ͼ�ش�

��1��ѹǿp1��p2 �����С����
��2����a+b���ȣ�c+d�� �����С����
��3���¶�t1��t2�� ����ߡ��͡���
��4������ӦΪ �ȷ�Ӧ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ָ����Ӧ�����ӷ���ʽ��ȷ����
A. �ữNaIO3��NaI�Ļ����Һ��I +IO3+6H+![]() I2+3H2O
I2+3H2O
B. ����Na2CO3��Һ��CaSO4���巴Ӧ��CO32+CaSO4![]() CaCO3+SO42
CaCO3+SO42
C. KClO������Һ��Fe(OH)3��Ӧ��3ClO+2Fe(OH)3![]() 2FeO42+3Cl+4H++H2O
2FeO42+3Cl+4H++H2O
D. ��ⱥ��ʳ��ˮ��2Cl+2H+![]() Cl2��+ H2��
Cl2��+ H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����0.1 mol��L-1NaHCO3��0.1 mol��L-1NaOH�����������õ���Һ�У����ӵ����ʵ���Ũ�ȹ�ϵ����ȷ������ ��
A��c��Na+����c��CO32-����c��HCO3-����c��H2CO3��
B��c��Na+����2��c��CO32-����c��HCO3-����c��H2CO3����
C��c��OH-����c��HCO3-����c��H+����2c��H2CO3��
D��c��Na+����c��H+����c��CO32-����c��HCO3-����c��OH-��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�Ka(HCOOH)=1.77��104��Ka(CH3COOH)=1.75��105��Kb(NH3��H2O) =1.75��105������˵����ȷ����
A. ��ͬ���pH��Ϊ3��HCOOH��CH3COOH��Һ���к�NaOH��������ͬ
B. 0.2 mol��L-1 HCOOH��0.1 mol��L-1 NaOH �������Ϻ�c(HCOO) + c(OH) < c(HCOOH) + c(H+)
C. Ũ�Ⱦ�Ϊ0.1 mol��L1�� HCOONa��NH4Cl ��Һ�������ӵ����ʵ���Ũ��֮�ͣ�ǰ�ߴ��ں���
D. ��CH3COONa��Һ��20��������30�棬��Һ��![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ��CuFeS2������ͭ������Ҫ�����ұ����ͭ��Ĺ����У�����һ����Ӧ�ǣ�2Cu2O+ Cu2S![]() 6Cu+SO2���ش��������⡣
6Cu+SO2���ش��������⡣
��1��Cu+�ļ۵��ӹ����ʾʽΪ__________________��Cu2O��Cu2S�Ƚϣ��۵�ϸߵ���_______��ԭ��Ϊ_____________________________________��
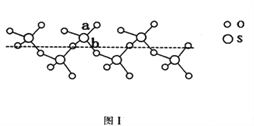
��2��SO2��SO3�ļ�����ȣ����Ǹ������____________������Һ̬SO3��ȴ��289.8Kʱ���̵õ�һ������״�����ṹ�Ĺ��壬��ṹ����ͼ1��ʾ���˹�̬SO3��Sԭ�ӵ��ӻ����������_______���ýṹ��S��O���������࣬һ�����Լ140pm����һ�����ԼΪ160pm���϶̵ļ�Ϊ_________����ͼ����ĸ����
��3�����ӻ�����CaC2��һ�־���ṹ����ͼ2��ʾ��д�������ʵĵ���ʽ_____���Ӹ����ӿ�������____________�ѻ���һ���������е�����ƽ����______����
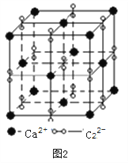

��4����������̼�ܽ����á�Fe���γɵ�һ�ּ�϶�����壬���ԣ��侧������ͼ3��ʾ��������ʵĻ�ѧʽΪ________���������ܶ�Ϊdg/cm3���������������̼ԭ�ӵľ���Ϊ____________________ pm���������ӵ�������ֵ��NA��ʾ��д������ʽ���ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������[ KAl(SO4)2��12H2O ]����ֽ����ˮ�ȷ���Ӧ�ù㷺���Դ������ķϾ���������мΪԭ��(��Ҫ�ɷ�ΪAl������������Fe��Mg����)�Ʊ������Ĺ�������ͼ��ʾ��

�ش��������⣺
(1)ԭ���ܽ�����з�Ӧ�����ӷ���ʽ��_________________________��
(2)Al(OH)3 ��ϡ���ᷴӦ�����ӷ���ʽ��__________________________��
(3)��ҺA��ͨ�����CO2����Ӧ�����ӷ���ʽ��_________________��
(4)Ϊ֤������B�к�������ijͬѧ������ʵ�飺ȡ��������B������ϡ����ʹ���ܽ⣬�۲쵽����ɫ�������ɡ�����Һ�м���___________����Һ������죬֤������B�к�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������и��仯�У���Ӧ��Ϊ�����µķ�Ӧ��A��C��D������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬E������Ϊ��ɫ��ζ��Һ�壬FΪ����ɫ��ĩ��GΪ��������ɫ���塣
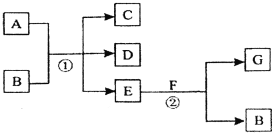
�ش��������⣺
(1)A��G�Ļ�ѧʽ�ֱ�Ϊ________________�� ________________��
(2)F��E��Ӧ�Ļ�ѧ����ʽΪ____________________________��
(3)д����Ӧ�ٵĻ�ѧ����ʽ____________________________________��
(4)�ڷ�Ӧ���У�ÿ����2.24 L����G(��״��)ʱ������F ___________g��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com