
·ÖĪö £Ø1£©ČÜŅŗÖŠĄė×ÓÅضČŌ½“ó£¬ČÜŅŗµÄµ¼µēŠŌŌ½Ē棻
£Ø2£©Č”Į½ÕÅpHŹŌÖ½·ÅŌŚ±ķĆęĆóÉĻ£¬²ā¶ØĮ½ÖÖČÜŅŗµÄpH£¬“×ĖįµÄpH“ó£»
£Ø3£©ÅضČĻąµČµÄHClČÜŅŗŗĶCH3COOHČÜŅŗ£¬“×ĖįÖŠĒāĄė×ÓÅØ¶ČŠ”£¬ÓėZn·“Ӧɜ³ÉĒāĘųµÄĖŁĀŹŠ”£»ĖįĻąĶ¬ÓėŠæ·“Ӧɜ³ÉµÄĒāĘųĻąĶ¬£®
½ā“š ½ā£ŗ£Ø1£©ČÜŅŗÖŠĄė×ÓÅضČŌ½“ó£¬ČÜŅŗµÄµ¼µēŠŌŌ½Ē棬ŃĪĖįĶźČ«µēĄė£¬ČÜŅŗÖŠĄė×ÓÅØ¶Č“óÓŚ“×ĖįÖŠĄė×ÓÅØ¶Č£¬ĖłŅŌŃĪĖįµÄµ¼µēŠŌĒ棬¼“ĶعżŃĪĖįµÄŠ”µĘÅŻ½ĻĮĮ£»
¹Ź“š°øĪŖ£ŗHClČÜŅŗ£»
£Ø2£©Č”Į½ÕÅpHŹŌÖ½·ÅŌŚ±ķĆęĆóÉĻ£¬ÓĆ½ą¾»²£Į§°ō·Ö±šÕŗČ”“ż²āŅŗµćµ½ŹŌÖ½µÄÖŠ¼ä£¬±äÉ«ŗó£¬Č»ŗóÓė±ČÉ«æØ±Č½Ļ£¬Č·¶ØĘäpH£¬ÓÉÓŚ“×Ėį²æ·ÖµēĄė£¬ČÜŅŗÖŠĒāĄė×ÓÅØ¶ČŠ”£¬ĖłŅŌ“×ĖįµÄpH“ó£»
¹Ź“š°øĪŖ£ŗČ”Į½ÕÅpHŹŌÖ½·ÅŌŚ±ķĆęĆóÉĻ£¬ÓĆ½ą¾»²£Į§°ō·Ö±šÕŗČ”“ż²āŅŗµćµ½ŹŌÖ½µÄÖŠ¼ä£¬Č»ŗóÓė±ČÉ«æØ±Č½Ļ£¬Č·¶ØĘäpH£»CH3COOHČÜŅŗ£»
£Ø3£©ÅضČĻąµČµÄHClČÜŅŗŗĶCH3COOHČÜŅŗ£¬“×Ėį²æ·ÖµēĄė£¬“×ĖįÖŠĒāĄė×ÓÅØ¶ČŠ”£¬ÓėZn·“Ӧɜ³ÉĒāĘųµÄĖŁĀŹŠ”£»ĪļÖŹµÄĮæÅضČĻąµČµÄHClČÜŅŗŗĶCH3COOHČÜŅŗ£¬Ģå»żĻąĶ¬Ź±£¬ĖįµÄĪļÖŹµÄĮæĻąĶ¬£¬ŌņÓėŠæ·“Ӧɜ³ÉµÄĒāĘųĻąĶ¬£»
¹Ź“š°øĪŖ£ŗ£¾£»=£®
µćĘĄ ±¾Ģāæ¼²éĮĖĢ½¾æĖįŠŌĒæČõµÄŹµŃé·½°øÉč¼Ę£¬ĢāÄæÄѶČÖŠµČ£¬²ąÖŲÓŚæ¼²éѧɜµÄŹµŃéĢ½¾æÄÜĮ¦ŗĶ·ÖĪöÄÜĮ¦£¬×¢Ņā°ŃĪÕ“×ĖįµÄµēĄėĢŲµć£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | H2RŹĒ¶žŌŖČõĖį£¬ĘäKal=1”Į10-2 | |
| B£® | µ±ČÜŅŗĒ”ŗĆ³ŹÖŠŠŌŹ±£¬c£ØNa+£©=2c£ØR2-£©+c£ØHR-£© | |
| C£® | NaHRŌŚČÜŅŗÖŠĖ®½āĒćĻņ“óÓŚµēĄėĒćĻņ | |
| D£® | ŗ¬Na2RÓėNaHRø÷0.1molµÄ»ģŗĻČÜŅŗµÄpH=7.2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | C-HÖ®¼äÖ»ŹĒsp2ŠĪ³ÉµÄ¦Ņ¼ü£¬C-CÖ®¼äÖ»ŹĒĪ“²Ī¼ÓŌӻƵÄ2p¹ģµĄŠĪ³ÉµÄ¦Š¼ü | |
| B£® | C-C¼äŹĒsp2ŠĪ³ÉµÄ¦Ņ¼ü£¬C-HÖ®¼äŹĒĪ“²Ī¼ÓŌӻƵÄ2p¹ģµĄŠĪ³ÉµÄ¦Š¼ü | |
| C£® | sp2ŌӻƹģµĄŠĪ³É¦Ņ¼ü”¢Ī“ŌӻƵÄ2p¹ģµĄŠĪ³É¦Š¼ü | |
| D£® | sp2ŌӻƹģµĄŠĪ³É¦Š¼ü”¢Ī“ŌӻƵÄ2p¹ģµĄŠĪ³É¦Ņ¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŹŅĪĀĻĀ£¬1 L pH=13µÄNaOHČÜŅŗÖŠ£¬ÓÉĖ®µēĄėµÄOH-ŹżÄæĪŖ0.1NA | |
| B£® | 2.0gH218OÓėD216OµÄ»ģŗĻĪļÖŠĖłŗ¬ÖŠ×ÓŹżĪŖNA | |
| C£® | 50ml 12mol/LŃĪĖįÓė×ćĮæMnO2¹²ČČ£¬×ŖŅʵĵē×ÓŹżĪŖ0.3NA | |
| D£® | ³£ĪĀ³£Ń¹ĻĀ£¬4.4gŅŅČ©Ėłŗ¬¦Ņ¼üŹżÄæĪŖ0.7NA |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
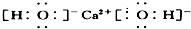
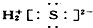

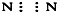

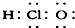
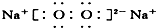
| A£® | ¢Ł¢Ś¢Ū¢Ü | B£® | ¢Ż¢Ž¢ß¢ą | C£® | ¢Ś¢Ū¢Ż¢Ž¢ß | D£® | ¢Ł¢Ü¢ą |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | RŹĒµŚČżÖÜĘŚŌŖĖŲ | |
| B£® | RŌŖĖŲµÄ×īøßÕż¼ŪÓėNŌŖĖŲ×īøßÕż¼ŪĻąĶ¬ | |
| C£® | RO3-ŗĶNO3-¾łÖ»Äܱ»»¹Ō£¬²»Äܱ»Ńõ»Æ | |
| D£® | RŗĶNĮ½ŌŖĖŲµÄµ„ÖŹ¶¼ŹĒæÕĘųµÄÖ÷ŅŖ³É·Ö |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2016-2017ѧğĢģ½ņŹŠøßŅ»ÉĻ9ŌĀµ÷ŃŠ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗŃ”ŌńĢā
¹ŲÓŚµČÖŹĮæµÄSO2ŗĶSO3µÄ±Č½Ļ£¬ÕżČ·µÄŹĒ
A£®Ėłŗ¬ŃõŌ×ÓµÄøöŹż±ČĪŖ2£ŗ3 B£®Ėłŗ¬ĮņŌ×ÓµÄøöŹż±ČĪŖ1£ŗ1
C£®Ėłŗ¬ĮņŌŖĖŲµÄÖŹĮæ±ČĪŖ5£ŗ4 D£®Ėłŗ¬Ō×ÓøöŹż±ČĪŖ3£ŗ4
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | H£ŗCl | B£® | H[£ŗO£ŗ]2- H | C£® | Mg2+ 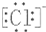 2 2 | D£® | Na+  |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com