

| ʵ���� | ����HCl��Һ�����(mL) | ����NaOH��Һ�����(mL) |
| 1 | 20.00 | 23.00 |
| 2 | 20.00 | 23.10 |
| 3 | 20.00 | 22.90 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ͬŨ�ȵ�CH3COOH��NaOH��Һ�������� |
| B��pH��2��HCl��pH��12��NaOH��Һ�������� |
| C��pH��3��HCl��pH��12��NaOH��Һ�������� |
| D��pH��2��CH3COOH��pH��12��NaOH��Һ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������Һϡ��100������ҺpH�Ĵ�С˳�ۣ��ܣ��٣��� |
| B���ۺֱܷ͢��õ�Ũ�ȵ�������Һ�кͣ�����������Һ��������ۣ��� |
| C������ڻ�ϣ����û����Һ��pH��3 |
| D���ں͢ۻ�Ϻ���Һ�ʼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����������ḻ��ú̿��Դ�����ٶ�ʯ�͵����� |
| B�����������غ������˽���������վ�������Դ���� |
| C��������ܶೡ�ݵ����ʹ�÷Ǿ��象Ĥ���Գ������̫���ܣ����ֵ�̼���� |
| D������ͣ������װ�������ʩ���ɽ�����β����CO��NOx��Ӧ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��c(OH��)=1��10��11mol��L��1���������� | B��pH=3��CH3COOH��Һ |
| C��pH=4��H2SO4��Һ | D��c(H+)=1��10��3mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
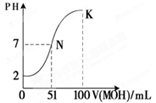
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2H2(g)+O2(g)��2H2O(g)����H1 |
| B��S(g)+O2(g)�� SO2 (g)����H1 |
C��C(s)+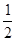 O2(g)�� CO(g)����H1 O2(g)�� CO(g)����H1 |
| D��H2(g)+Cl2(g) �� 2HCl(g)����H1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
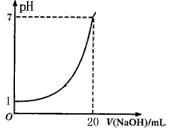
| A����Һ�����Ũ��Ϊ0��1 mol/L |
| B��NaOH��Һ��Ũ��Ϊ0��05 mol/L |
| C���ζ��յ�ʱ�����Ӽ�ʽ�ζ��ܶ���������NaOH��ҺŨ�Ȼ�ƫ�� |
| D��ָʾ����ɫʱ��˵��������NaOHǡ����ȫ��Ӧ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com