
ij����С�����Mg��CO2�ķ�Ӧԭ����̽��Mg��NO2�ķ�Ӧ����������С��ͨ��ʵ��ȷ��Mg����NO2��ȼ�գ����Թ������������ּ��裺
I.����Ϊ���������ΪMgO II.����Ϊ��______________III.����Ϊ��______________
��ش��������⣺������Ϣ��2NO2+2NaOH=NaNO3+NaNO2+H2O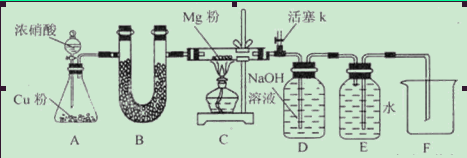
��1����ͼ���Ӻ�������װҩƷǰ��μ���װ�õ�������_____________________________
��2��װ��B��ʢװ�ĸ���������ǣ�����ţ�___________________
��Ũ���� ����ˮCaCl2 �ۼ�ʯ�� ������������
��3����ʼ����k,��A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ����_________________________
��4��E���ռ��������������������ܶ���14,��������__________________
��5��ʵ��õ�����������������ʵ��ǰMg��������1.5���������__________________������C�з����Ļ�ѧ��Ӧ����ʽ��_______________________________________________
��6����ʵ���д�������ȱ�ݣ��Ľ���ʩ��_______________________________________��
��.�������ΪMg3N2 ��.�������ΪMgO��Mg3N2 ��1���رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á� ��2���ڢ� ��3���ž�װ���п�������ֹ��������ʵ�顣
��4��N2 ��5������� 4Mg+2NO2 4MgO+N2 3Mg+N2
4MgO+N2 3Mg+N2 Mg3N2
Mg3N2
��6������K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
���������������NO2�к���N��OԪ�أ�����Mg������ȼ�ղ��������MgO��Mg3N2�����ǵĻ��������.�������ΪMg3N2 ����.�������ΪMgO��Mg3N2��1����ͼ���Ӻ�������װҩƷǰװ�õ������Լ��鷽���ǣ��رշ�Һ©�������ͻ���K����������ĩ�˲���ˮ�У�����ƿ����C����Ӧ�ܣ��ȣ��������ܿ������ݣ�ֹͣ���ȣ��������н���һ��ˮ������ʾ���������á���2��Cu��Ũ���ᷴӦ����������NO2���������壬����ʹ�ü��Ը����������ų� �ۼ�ʯ�ң�U�ι�װ���ǹ�����������Ũ������Һ�壬����ʹ�ã��ų���Ũ���ᡣ��װ��B��ʢװ�ĸ���������Ǣ���ˮCaCl2 �����������ס���3����ʼ����k,��A�з�Ӧ����һ��ʱ�䣬��C�г�������ɫ����رջ���k���ٵ�ȼC���ƾ��ƣ�ͬʱD�м�Һʢ�ĺ�������������Ŀ�����ž�װ���п�������ֹ��������ʵ�顣��4��E���ռ��������������������ܶ���14,��Է�������Ϊ14��2=28����������N2.��5������ȫת��ΪMgO����Ӧ��Ĺ��������Ƿ�Ӧǰ��(24+16)��24=1.67��.����ȫת��ΪMg3N2����Ӧ��Ĺ��������Ƿ�Ӧǰ��(24��3+14��2)��(24��3)=1.39����ʵ��õ�����������������ʵ��ǰMg��������1.5�������Եõ�����MgO��Mg3N2�Ļ����ʼ�����������C�з����Ļ�ѧ��Ӧ����ʽ��4Mg+2NO2 4MgO+N2 3Mg+N2
4MgO+N2 3Mg+N2 Mg3N2����6����ʵ���д�������ȱ�ݣ�����û��β������װ�á��Ľ���ʩ�ǻ���K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
Mg3N2����6����ʵ���д�������ȱ�ݣ�����û��β������װ�á��Ľ���ʩ�ǻ���K���ĵ����ܲ���NaOH��Һ�У�����β�����ա�
���㣺����̽��Mg��NO2�ķ�Ӧ���������ijɷ֡�


 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��˾ƥ��(����ˮ����,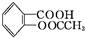 )��������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ�,�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ����(���ǻ�������)�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ��,�Ʊ�����������������:
)��������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ�,�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ����(���ǻ�������)�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ��,�Ʊ�����������������:
������+ˮ����
 �ֲ�Ʒ
�ֲ�Ʒ
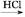
 ����ˮ����
����ˮ����
(��˾ƥ��) ���۵�
���۵�
��Ҫ�Լ��Ͳ�Ʒ����������
| ���� | ��Է������� | �۵��е�(��) | ˮ |
| ˮ���� | 138 | 158(�۵�) | �� |
| ������ | 102 | 139.4(�е�) | ��Ӧ |
| ����ˮ���� | 180 | 135(�۵�) | �� |
 ��
�� ��
�� ��
�� ��
�� ����ˮ����
����ˮ����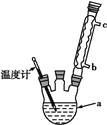
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
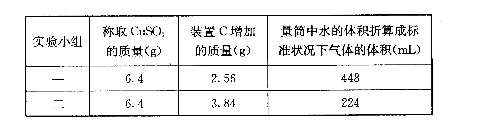
ij�С���CuSO4�ֽ�����CuO��������3�ֿ����Խ��м���,��ͨ��ʵ��ȷ����������ʵ�����
(1)�����3�ֿ�����Ϊ����������;������������;��SO2��SO3��O2��
(2)Ϊȷ��SO2��SO3��O2�����ʵ���֮��,����ѡ����������������ʵ��,������������ҵķ������Ӹ�װ�á�˳��Ϊ:��������������B��(��֪SO3����Ũ����) 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����к�̼�����Ԫ�ء���ѧ��ȤС���ij������Ʒ������̽��������Ҫ��ش��������⡣
��.������̼����Ԫ�صĶ��Լ���
����ͼװ�ý���ʵ��(�г���������ȥ,��ӿ���ĸ����)����ʵ��̼����Ԫ�صļ��顣
(1)����X��������������;װ�âۢ����Լ���ͬ,װ�â���ʢ�ŵ��Լ����� ������
(2)д�����з�Ӧ�Ļ�ѧ����ʽ����������������������������������������
(3)�������װ�âۢܢ�,�ܷ�ȷ��������Ʒ��̼Ԫ�صĴ���?��������,������������������������������������������������
��.������̼�������������IJⶨ
(4)��ͬѧ��Ϊ,����װ�ÿ��Դ��Բⶨ��Ʒ��̼�ĺ�������ȡ��Ʒw1 g����ʵ��,��ַ�Ӧ��,���װ�â������ɵij���Ϊw2 g,����Ʒ��̼����������Ϊ������(�ú�w1��w2��ʽ�ӱ�ʾ)��
(5)��ͬѧ��Ϊ,��һ������Ʒ��ַ�Ӧ��,��װ�â��м�������Ȼ�����Һ,���ݳ����������Լ�����Ʒ�������������,�˷�����õĽ��������(�ƫ��ƫС��);��Ҫ�����Ԫ�غ����IJⶨ����,�ڲ��ı�ʵ��ԭ����ǰ����,���Բ�ȡ��һ�ִ�ʩ������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ���ɵ�����ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO3��SO2��O2�е�һ�֡����ֻ����֡�ij��ѧ����С�����̽����ʵ�飬�ⶨ��Ӧ������SO3��SO2��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
��������롿
����I������ͭ���ȷֽ���������ijɷֿ���ֻ��SO3һ�֣�
���������ͭ���ȷֽ���������ijɷֿ���ֻ��_______���֡�
���������ͭ���ȷֽ���������ijɷֿ��ܺ���_______���֡�
��ʵ��̽����
��֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1����װ̽��ʵ���װ�ã����������ҵķ��������ӿ�����˳��Ϊ�١��������ޡ��ݡ�____��_____��_____��______���ڡ�(��ӿ����)
��2����ʵ�����ʱװ��B����Ͳû���ռ���ˮ����֤������_______(�I������)��ȷ��
��3��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ���������������Ҳ��
ͬ���������£�
��ͨ�����㣬�ƶϳ��ڵ�һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺_____________________________________________________________;
�ڶ�С�飺_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��̼���ƣ�2Na2CO3��3H2O2����һ�ּ�ϴ�ӡ�Ư�ס�ɱ����һ�����ϵƯ�����þ������Na2CO3��H2O2��˫�����ʡ�����ͼ-2װ���Ʊ���̼���ƣ�����ˮԡ�г�ַ�Ӧ��ͼ-1���̿ɻ�ù�̼���Ʋ�Ʒ��

��1����ѹ��Һ©����֧�ܵ������� ��
��2���Ʊ���̼���ƵĹؼ��� ��
��3��������ƹ�̼���Ƶ�ˮ�к��������ӣ�����������ϴ�Ӽ���ȥ��������������ȫʧȥɱ�����á��Է������е�ԭ��д������һ�ּ��ɣ��÷���ʽ��ʾ����________________________________��
��4��ij��ѧѧϰС��Ϊ�˶���̽�������Ӷ���������Ư���IJ���Ӱ�죬ȡ��Ư��100mL������25g FeCl3���壬����������ɫ��ζ���壬������ƿ�ռ����塣��ѡ�������Լ���ʵ����Ʒ�������ɷֵ�̽�����̣�0��1mol/LNaOH��Һ��8��0mol/LNaOH��Һ������ʯ��ˮ��0��01mol/LKMnO4��Һ��BaCl2ϡ��Һ��Ʒ����Һ������ˮ��ľ�����ƾ��ơ����ϴ��ƿ��
��������裺�Ը�����ɷ�����������衣
����1��������O2�� ����2��������______________�� ����3��������CO2��
����Ʒ��������ʵ�鷽��֤����ļ��裬���±������ʵ�鲽�衢Ԥ����������ۣ�
| ʵ�鲽�� | Ԥ����������� |
| ����������ͨ��ʢ��_______��________��ϴ��ƿ�У�________________________�� | ��________________________ ��________________________ ��________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����(�Ҷ���)������ԭ���ͳ����������ڽ������⡢֯��Ư��ϡ��������һ���Ʊ�����(��2���ᾧˮ)�Ĺ����������ң�
�ش��������⣺
��1��CO��NaOH��һ�������ºϳɼ����ơ������Ƽ�������Ļ�ѧ��Ӧ����ʽ�ֱ�Ϊ�� �� ��
��2�����Ʊ������������ι��˲��������˲����ٵ���Һ�� �������� �����˲����ڵ���Һ��_ �� �������� ��
��3�����չ����Тۺܵ͢�Ŀ���� ��
��4�����˽�������������ֱ���������ữ�Ʊ����ᡣ�÷�����ȱ���Dz�Ʒ���������к��е�������Ҫ�� ��
��5���ᾧˮ�ϲ����Ʒ�Ĵ����ø�����ط��ⶨ��
���������Ʒ0.250 g����ˮ����0.0500 mol��L-1������KMnO4��Һ�ζ�����dz�ۺ�ɫ�����ʣ�����KMnO4��Һ15.00 mL����Ӧ�����ӷ���ʽΪ ��
��ʽ����ó�Ʒ�Ĵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��̼����(2Na2CO4��3H2O)��һ������Ч����Ư��ɱ������������������������Ⱦ���ص㣬������Ӧ����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������С����д���Ʊ���̼���ƿɽ��������ɱ����������������£�
�Իش��������⣺
��1����̼��������ˮ��������Һ�� (����ԡ��������ԡ������ԡ�)���������ӷ���ʽ��ʾ��______________________��
��2����������Ҫ�IJ��������� (��д��������)��
��3����ҵ�����г���������NaCl��ijУ��ѧ����С�������ͼ��ʾװ�ã��ⶨ��ҵ������Na2CO3�ĺ�����
��Ҫ���鹤ҵ���������ʵĴ��ڣ����ѡ�������Լ��е�__________(ѡ�����)��
a. ����������Һ b. ϡ���� c. ���軯����Һ d. ��������Һ
�ڼ���װ��B�����Եķ����ǣ�����������©���������������н����ɼк�©��ע��һ������ˮ��ʹ©���ڵ�ˮ�����ƿ�ڵ�ˮ�棬ֹͣ��ˮ�����۲쵽 ˵��װ�ò�©����
��װ��A�������� ��װ��C�е��Լ�Ϊ ��
��ijͬѧ��Ϊ��Dװ�ú�Ӧ������Eװ��(װ���ʵ��Լ�)������Ϊ�Ƿ��Ҫ? (ѡ���Ҫ������Ҫ��)���жϵ������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԡ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ��
����1������ˮ����Ũ����ȥ����
����2������ȥ���κ��ĸҺ�ữ��ͨ��������������ʹBr��ת��ΪBr2��
����3������2����ˮ��Һ��ͨ���ȿ�����ˮ���������嵥�ʴ���ʢ�ж�������ˮ��Һ��������
����4�������������ͨ��������������ʹBr��ת��ΪBr2
����5�������Ȼ�̼��ȡ�嵥�ʣ�����Һ������ô��塣
��1������3�еķ�Ӧ�����ӷ���ʽ ��
��2������2���Ѿ��Ƶ����壬��Ҫ���в���3�Ͳ���4��ԭ���� ��Ԫ�ء�
��3������5����ȡ�ͷ�Һ����Ҫ����Ҫ��������Ϊ ��
��4��������ͼʵ��װ�þ��ƴ��塣
��ͼ����ȴˮӦ��B�� �ڽ���(�a����b��) ��
��C�мӱ���Ŀ���ǽ��£�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com