��������1����������2��5-���������̼�ܣ��ڸ�������̼��������̼̼�������ɣ�
��2��̼ԭ������10���ڵ�����������ͬ���칹���У�һ�ȴ�����ͬ���칹�壬˵����������ֻ��һ�ֵ�Ч��ԭ�ӣ����ݵ�Ч��ԭ�ӵ��жϷ������ش�
��3���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ��˵��A�к��в����ͼ���A��һԪ���ᣬ����A����-COOH�����ݺ�̼����������Ϊ50.0%��⣻
��4����ƿ��������4.32g��ΪAg�������ʵ���n=
=0.04mol������R-CHO��2Ag�����۽�𣻣�5��Ũ���������ˮ�ԣ�������������10.8g��˵����Ӧ�����к�ˮ10.8g��ͨ����������ͭ�����ڷ�����ӦCuO+CO
Cu+CO
2����������������3.2g����Ϸ���ʽ���ò������ɼ���CO�����ʵ�����ͨ����ʯ��ʱ����ʯ�ҵ�����������17.6g�ɼ�����CO
2�����ʵ�������ȥCO��CuO��Ӧ���ɵ�CO
2������Ϊ�л���ȼ������CO
2������������Ԫ���غ�����л����к���C��H��O�����ʵ�����������û�ѧʽ����Ϸ���ʽ�����Ʒ�Ӧ�����ʵ���ȷ�����������༰��Ŀ���ݴ���д�л�������ƣ�
���
�⣺��1��2��5-���������̼��Ϊ��C-C��C��-C-C-C��C��-C���ڸ�̼��������̼̼���������Եõ�Ȳ������Ȳ���Ľṹ��ʽΪ��
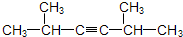
������CH
3��
2CHC��CCH��CH
3��
2��
�ʴ�Ϊ����CH
3��
2CHC��CCH��CH
3��
2��
��2�����������У�ͬһ��̼�ϵ���ԭ�ӵ�Ч������ͬһ��̼ԭ���ϵ���ԭ�ӵ�Ч�����о���ԳƵ�̼ԭ���ϵ���ԭ�ӵ�Ч��������һ��ȡ����ֻ��һ�֣�˵����������ֻ��һ�ֵ�Ч��ԭ�ӣ���̼ԭ����n��10������������ͬ���칹���У���һ��ȡ����ֻ��һ�ֵ������ֱ��ǣ����顢���顢2��2-���������Լ�2��2��3��3-�ļ����飬
�ʴ�Ϊ��4��
��3�����һԪ�������ʽΪC
nH
mO
2���ɺ�̼����������Ϊ50%���ã�12n=m+32���������塢�廯�ⶼ���Ը�A��ӳɷ�Ӧ��˵��A�к��в����ͼ������ۿɵã�n=3��m=4����C
3H
4O
2��Ϊ��ϩ�ᣬ��ṹ��ʽΪ��CH
2=CH-COOH��
�ʴ�Ϊ��CH
2=CHCOOH��
��4����ƿ��������4.32g��ΪAg�������ʵ���n=
=0.04mol��10g11.6%��ijȩ��Һȩ�����ʵ���Ϊ10��11.6%=1.16g��
��ΪһԪȩ����һԪȩ�����ʵ���Ϊx��
�� R-CHO��2Ag
1mol 2mol
x 0.04mol x=0.02mol��
RCHO��Ħ������M=
=
=58g/mol������ȩͨʽC
nH
2nO��Ϊ��ȩCH
3CH
2CHO��
��Ϊ��Ԫȩ ����
OHC-RCHO��4Ag
1mol 4mol
0.01mol 0.04mol
���Ԫȩ��Ħ������M=
=
=116g/mol��-CHOΪ29g/mol����R=116-29��2=58����������ͬʱ���ų���ȩ�Ŀ����ԣ�
�ʴ�Ϊ��CH
3CH
2CHO��
��5���л���ȼ������ˮ10.8g��ˮ�����ʵ���Ϊ��
=0.6mol��
���л���ȼ�����ɵ�CO����Ϊx��
��CuO+CO
Cu+CO
2����̬���ء�m
28g 16g
x 3.2g
����x=
=5.6g��CO�����ʵ���Ϊ��
=0.2mol��
����̼Ԫ���غ��֪CO��CuO��Ӧ���ɵ�CO
2�����ʵ���Ϊ0.2mol������Ϊ��0.2mol��44g/mol=8.8g��
�л���ȼ�����ɵ�CO
2������Ϊ��17.6g-8.8g=8.8g�����ʵ���Ϊ��
=0.2mol��
����̼Ԫ���غ��֪��1mol�л��ﺬ��̼ԭ�����ʵ���Ϊ��
mol=2mol��
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ��
mol=6mol
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ��
=2mol��
�����л���ķ���ʽΪ��C
2H
6O
2��9.2g ��Ϊ0.4mol��0.2mol���л�����0.4mol��ǡ�÷�Ӧ���л����к���2���ǻ������л���Ľṹ��ʽΪOHCH
2CH
2OH�����л��������Ϊ�Ҷ�����
�ʴ�Ϊ���Ҷ�����

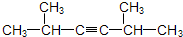 ������CH3��2CHC��CCH��CH3��2��
������CH3��2CHC��CCH��CH3��2��

 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�