£Ø2013?±£¶ØŅ»Ä££©ÖŲøõĖį¼Ų£ØK
2Cr
2O
7£©ŹĒ¹¤ŅµÉś²śŗĶŹµŃéŹŅµÄÖŲŅŖŃõ»Æ¼Į£®ŹµŃéŹŅÄ£Äā¹¤ŅµÉś²śÖŲøõĖį¼ŲŹ±ÓĆøõĢśæó£ØÖ÷ŅŖ³É·ŻĪŖFeO?Cr
2O
3£©”¢“æ¼ī”¢ÉÕ¼ī”¢ĀČĖį¼ŲµČĪŖŌĮĻĻČÖʵĆøõĖįÄĘ£ØNa
2CrO
4£©£¬Éę¼°µÄÖ÷ŅŖ·“Ó¦ŹĒ6FeO?Cr
2O
3+24NaOH+7KClO
3 12Na
2CrO
4+3Fe
2O
3+7KCl+12H
2O£¬Č»ŗó½«øõĖįÄĘ×Ŗ»ÆĪŖK
2Cr
2O
7£®ĘäÖ÷ŅŖ¹¤ŅÕČēĻĀ£ŗ
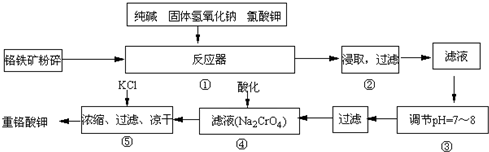
ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŌŚ·“Ó¦Ę÷¢ŁÖŠ£¬ÓŠNa
2CrO
4Éś³É£¬Ķ¬Ź±Fe
2O
3×Ŗ±äĪŖNaFeO
2£¬ŌÓÖŹSiO
2ӢAl
2O
3Óė“æ¼ī·“Ó¦×Ŗ±äĪŖæÉČÜŠŌŃĪ£¬Š“³öŃõ»ÆĀĮÓėĢ¼ĖįÄĘ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
Al
2O
3+Na
2CO
32NaAlO
2+CO
2ӟ
Al
2O
3+Na
2CO
32NaAlO
2+CO
2ӟ
£®
£Ø2£©NaFeO
2ÄÜĒæĮŅĖ®½ā£¬ŌŚ²Ł×÷¢ŚÉś³É³Įµķ¶ų³żČ„£¬Š“³öøĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ
NaFeO2+2H2O=Fe£ØOH£©3”ż+NaOH
NaFeO2+2H2O=Fe£ØOH£©3”ż+NaOH
£®
£Ø3£©²Ł×÷¢ŪµÄÄæµÄŹĒŹ²Ć“£¬ÓĆ¼ņŅŖµÄĪÄ×ÖŗĶ»Æѧ·½³ĢŹ½ĖµĆ÷£ŗ
ÓÉÓŚ¹čĖįÄĘŗĶĘ«ĀĮĖįÄĘ·¢ÉśĖ®½ā£ŗSiO
32-+2H
2O
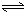
H
2SiO
3+2OH
-£Ø»ņSiO
32-+H
2O
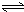
HSiO
3-+OH
-£¬HSiO
3-+H
2O
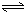
H
2SiO
3+OH
-£¬AlO
2-+H
2O
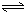
Al£ØOH£©
3+OH
-£©£¬½µµĶpHÖµÓŠĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬µ±pHµ÷µ½7”«8Ź±£¬Ź¹ĖüĆĒĖ®½āĶźČ«
ÓÉÓŚ¹čĖįÄĘŗĶĘ«ĀĮĖįÄĘ·¢ÉśĖ®½ā£ŗSiO
32-+2H
2O
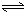
H
2SiO
3+2OH
-£Ø»ņSiO
32-+H
2O
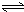
HSiO
3-+OH
-£¬HSiO
3-+H
2O
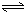
H
2SiO
3+OH
-£¬AlO
2-+H
2O
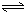
Al£ØOH£©
3+OH
-£©£¬½µµĶpHÖµÓŠĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬µ±pHµ÷µ½7”«8Ź±£¬Ź¹ĖüĆĒĖ®½āĶźČ«
£®
£Ø4£©²Ł×÷¢ÜÖŠ£¬Ėį»ÆŹ±£¬CrO
42-×Ŗ»ÆĪŖCr
2O
72-£¬Š“³öĘ½ŗā×Ŗ»ÆµÄĄė×Ó·½³ĢŹ½£ŗ
2CrO
42-+2H
+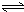
Cr
2O
72-+H
2O
2CrO
42-+2H
+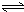
Cr
2O
72-+H
2O
£®
£Ø5£©³ĘČ”ÖŲøõĖį¼ŲŹŌŃł2.5000gÅä³É250mLČÜŅŗ£¬Č”³ö25.00mLÓŚµāĮæĘæÖŠ£¬¼ÓČė10mL 2mol/LH
2SO
4ČÜŅŗŗĶ×ćĮæµā»Æ¼Ų£ØøõµÄ»¹Ō²śĪļĪŖCr
3+£©£¬·ÅÓŚ°µ“¦5min£¬Č»ŗó¼ÓČė100mLĖ®£¬¼ÓČė3mLµķ·ŪÖøŹ¾¼Į£¬ÓĆ0.1200mol/LNa
2S
2O
3±ź×¼ČÜŅŗµĪ¶ØI
2+2S
2O
32-=2I
-+S
4O
62-£®
¢ŁÅŠ¶Ļ“ļµ½µĪ¶ØÖÕµćµÄŅĄ¾ŻŹĒ£ŗ
µ±µĪ¼Ó×īŗóŅ»µĪĮņ“śĮņĖįÄĘČÜŅŗŹ±£¬ČÜŅŗĄ¶É«ĶŹČ„£¬°ė·ÖÖÓÄŚ²»±äÉ«
µ±µĪ¼Ó×īŗóŅ»µĪĮņ“śĮņĖįÄĘČÜŅŗŹ±£¬ČÜŅŗĄ¶É«ĶŹČ„£¬°ė·ÖÖÓÄŚ²»±äÉ«
£»
¢ŚČōŹµŃéÖŠ¹²ÓĆČ„Na
2S
2O
3±ź×¼ČÜŅŗ40.00mL£¬ŌņĖłµĆ²śĘ·ÖŠµÄÖŲøõĖį¼ŲµÄ“æ¶ČĪŖ£ŗ
94.08%
94.08%
£ØÉčK
2Cr
2O
7µÄĦ¶ūÖŹĮæĪŖ294g?mol
-1£¬Õūøö¹ż³ĢÖŠĘäĖüŌÓÖŹ²»²ĪÓė·“Ó¦£©£®





 ·¢Éśõ„»Æ·“Ó¦æÉÉś³É·Ö×ÓŹ½ĪŖC18H22O4µÄõ„£¬½įŗĻÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹŅŌ¼°ĢāÄæŅŖĒó½ā“šøĆĢā£®
·¢Éśõ„»Æ·“Ó¦æÉÉś³É·Ö×ÓŹ½ĪŖC18H22O4µÄõ„£¬½įŗĻÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹŅŌ¼°ĢāÄæŅŖĒó½ā“šøĆĢā£® ·¢Éśõ„»Æ·“Ó¦æÉÉś³É·Ö×ÓŹ½ĪŖC18H22O4µÄõ„£¬
·¢Éśõ„»Æ·“Ó¦æÉÉś³É·Ö×ÓŹ½ĪŖC18H22O4µÄõ„£¬ ·¢Éśõ„»Æ·“Ó¦£¬·½³ĢŹ½ĪŖ
·¢Éśõ„»Æ·“Ó¦£¬·½³ĢŹ½ĪŖ £¬
£¬ £»
£»



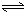 H2SiO3+2OH-£Ø»ņSiO32-+H2O
H2SiO3+2OH-£Ø»ņSiO32-+H2O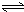 HSiO3-+OH-£¬HSiO3-+H2O
HSiO3-+OH-£¬HSiO3-+H2O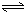 H2SiO3+OH-£¬AlO2-+H2O
H2SiO3+OH-£¬AlO2-+H2O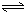 Al£ØOH£©3+OH-£©£¬½µµĶpHÖµÓŠĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬µ±pHµ÷µ½7”«8Ź±£¬Ź¹ĖüĆĒĖ®½āĶźČ«
Al£ØOH£©3+OH-£©£¬½µµĶpHÖµÓŠĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬µ±pHµ÷µ½7”«8Ź±£¬Ź¹ĖüĆĒĖ®½āĶźČ«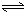 H2SiO3+2OH-£Ø»ņSiO32-+H2O
H2SiO3+2OH-£Ø»ņSiO32-+H2O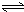 HSiO3-+OH-£¬HSiO3-+H2O
HSiO3-+OH-£¬HSiO3-+H2O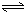 H2SiO3+OH-£¬AlO2-+H2O
H2SiO3+OH-£¬AlO2-+H2O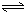 Al£ØOH£©3+OH-£©£¬½µµĶpHÖµÓŠĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬µ±pHµ÷µ½7”«8Ź±£¬Ź¹ĖüĆĒĖ®½āĶźČ«
Al£ØOH£©3+OH-£©£¬½µµĶpHÖµÓŠĄūÓŚĘ½ŗāĻņÕż·“Ó¦·½ĻņŅĘ¶Æ£¬µ±pHµ÷µ½7”«8Ź±£¬Ź¹ĖüĆĒĖ®½āĶźČ«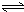 Cr2O72-+H2O
Cr2O72-+H2O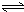 Cr2O72-+H2O
Cr2O72-+H2O