
ĻĀĮŠÓŠ¹ŲŹµŃé²Ł×÷”¢ĻÖĻóŗĶ½āŹĶ»ņ½įĀŪ¶¼ÕżČ·µÄ£Ø £©
| Ń”Ļī | ŹµŃé²Ł×÷ | ĻÖĻó | ½āŹĶ»ņ½įĀŪ |
| ¢Ł | ¹żĮæµÄ | ČÜŅŗ³ŹŗģÉ« | Ļ” |
| ¢Ś | AgI³ĮµķÖŠµĪČėĻ” | ÓŠ°×É«³Įµķ³öĻÖ |
|
| ¢Ū | Al²²åČėĻ” | ĪŽĻÖĻó |
|
| ¢Ü | ÓĆ²£Į§°ōÕŗČ”ÅØ°±Ė®µćµ½ŗģÉ«ŹÆČļŹŌÖ½ÉĻ | ŹŌÖ½±äĄ¶É« | ÅØ°±Ė®³Ź¼īŠŌ |
| ¢Ż | ½«ÅØĮņĖįµĪµ½ÕįĢĒ±ķĆę | ¹ĢĢå±äŗŚÅņÕĶ | ÅØĮņĖįÓŠĶŃĖ®ŠŌŗĶĒæŃõ»ÆŠŌ |
| ¢Ž | ½«×ćĮæµÄ |
| 2MnO4£+7H2O2+6H+=2Mn2++6O2”ü+10H2O |
| ¢ß | ½«Ņ»Š”æé | ²śÉśĘųÅŻ |
|
| ¢ą | ½«Ė®ÕōĘųĶعż×ĘČȵÄĢś·Ū | ·ŪÄ©±äŗģ | ĢśÓėĖ®ŌŚøßĪĀĻĀ·“Ó¦ |
A£®¢Ł¢Ü¢Ż¢Ž¢ß B£®¢Ü¢Ż C£®¢Ü¢Ż¢ß D£®¢Ł¢Ż

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
A”¢30 g SiO2ŗ¬ÓŠNAøöSi£O¹²¼Ū¼ü
B”¢1 L 0.2mol·L£1 Al2(SO4)3ČÜŅŗÖŠµÄĄė×Ó×ÜŹżĪŖNA
C”¢±ź×¼×“æöĻĀ£¬22.4 L H2OĖłŗ¬Ō×ÓøöŹż“óÓŚ3NA
D”¢ŗ¬4 mol HClµÄÅØŃĪĖįøś×ćĮæMnO2¼ÓČČ·“Ó¦æÉÖʵĆCl2µÄ·Ö×ÓŹżĪŖNA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
æÉÓėBa£ØOH£©2£ØŅŗĢ¬£©”¢CuSO4£Ø¹ĢĢ¬£©”¢“æCH3COOHČżÖÖĪļÖŹ¹éÓŚŅ»ĄąµÄĪļÖŹŹĒ
A£®C2H5OH£ØČÜŅŗ£© B£®HCl£ØĘųĢ¬£© C£®Ė®ĆŗĘų D£®¶¹½¬
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻąĶ¬ĪļÖŹµÄĮæµÄCO2ŗĶO2£¬ĘäĖłŗ¬Ō×ÓŹżÄæÖ®±ČĪŖ______________£¬Ėłŗ¬ŃõŌ×ÓŹżÄæÖ®±ČĪŖ________________£¬ÖŹĮæĻąĶ¬µÄCO2ŗĶO2£¬ĘäĪļÖŹµÄĮæÖ®±ČĪŖ________________£»ĻąĶ¬Ģõ¼žĻĀ£¬Ķ¬Ģå»żµÄCO2ŗĶO2 Ęä·Ö×ÓŹżÖ®±ČĪŖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪĄÉś²æ·¢³ö¹«øę£¬×Ō2011Äź5ŌĀ1ČÕĘš£¬½ūÖ¹ŌŚĆę·ŪÉś²śÖŠĢķ¼Ó¹żŃõ»ÆøĘ£ØCaO2£©µČŹ³Ę·Ģķ¼Ó¼Į”£ĻĀĮŠ¶Ō¹żŃõ»ÆøʵĊšŹö“ķĪóµÄŹĒ
A£®CaO2¾ßÓŠŃõ»ÆŠŌ£¬¶ŌĆę·ŪæÉÄܾßÓŠŌö°××÷ÓĆ
B£®CaO2ŗĶĖ®·“Ó¦Ź±£¬Ćæ²śÉś1 mol O2×ŖŅʵē×Ó2 mol
C£®CaO2ÖŠŅõŃōĄė×ÓµÄøöŹż±ČĪŖ2”Ć1
D£®CaO2ŗĶCO2·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2CaO2+2CO2 £½2Ca CO3+O2
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŽÉ«ČÜŅŗæÉÄÜÓÉK2CO3”¢MgCl2”¢NaHCO3”¢BaCl2ČÜŅŗÖŠµÄŅ»ÖÖ»ņ¼øÖÖ×é³É”£ĻņČÜŅŗÖŠ¼ÓČėÉÕ¼īČÜŅŗ³öĻÖ°×É«³Įµķ£¬¼ÓČėĻ”ĮņĖįŅ²³öĻÖ°×É«³Įµķ²¢·Å³öĘųĢ唣¾Ż“Ė·ÖĪö£¬ĻĀĮŠÅŠ¶ĻÖŠÕżČ·µÄŹĒ£Ø””””£©
¢ŁæĻ¶ØÓŠBaCl2”” ¢ŚæĻ¶ØÓŠMgCl2”” ¢ŪæĻ¶ØÓŠNaHCO3””
¢ÜæĻ¶ØÓŠNa2CO3»ņNaHCO3””¢ŻæĻ¶ØƻӊMgCl2
A£®¢Ł¢Ś¢Ū B£®¢Ś¢Ü C£®¢Ł¢Ū D£®¢Ł¢Ū¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĮņĢśæóÓÖ³Ę»ĘĢśæó£¬ŹĒÉś²śĮņĖįµÄŌĮĻ£¬ĘäÖ÷ŅŖ³É·ÖĪŖFeS2”£850”ę~950”ꏱ£¬ĮņĢśæóŌŚæÕĘųÖŠģŃÉÕ£¬æÉÄÜ·¢ÉśĻĀĮŠ·“Ó¦£ØÉčæÕĘųÖŠN2ÓėO2Ģå»ż±ČĪŖ4”Ć1£©£ŗ
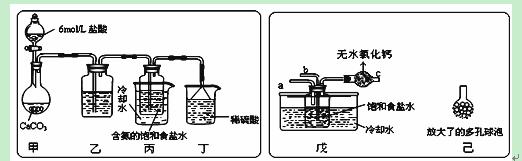
3FeS2 + 8O2 = Fe3O4 + 6SO2 ¢Ł
4FeS2 + 11O2 = 2Fe2O3 + 8SO2 ¢Ś
£Ø1£©ŗ¬Įņ35%µÄĮņĢśæóѳʷ£ØŌÓÖŹ²»ŗ¬Įņ£©£¬ĘäFeS2µÄŗ¬ĮæĪŖ_________________”£
£Ø2£©Éč1 t“æ¾»µÄFeS2°“¢ŚŹ½ĶźČ«·“Ó¦£¬²śÉś±ź×¼×“æöĻĀSO2__________ m3”£
£Ø3£©ĪŖŹ¹FeS2ģŃÉÕĶźČ«Éś³ÉFe2O3£¬¹¤ŅµÉĻŹ¹ÓĆ¹żĮææÕĘų£¬µ±æÕĘų¹żĮæ20%Ź±£¬ĖłµĆĀÆĘųÖŠSO2µÄĢå»ż·ÖŹżĪŖ¶ąÉŁ£æ ”£
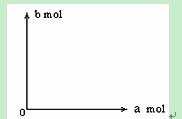
£Ø4£©480 g“æ¾»µÄFeS2ŌŚæÕĘųÖŠĶźČ«·“Ó¦£¬ČōĖłµĆ¹ĢĢåÖŠ£¬FeŗĶOµÄĪļÖŹµÄĮæÖ®±Čn£ØFe£©”Ćn£ØO£© =4”Ća£¬“ĖŹ±ĻūŗÄæÕĘųĪŖbmol”£
¢ŁŹŌŠ“³öbÓėaµÄ¹ŲĻµŹ½£ŗ___________________”£
¢Ś²¢×÷³öbÓėaµÄ¹ŲĻµĒśĻß”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
NA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
A£®32 gŃõĘųÓė×ćĮæļ®ĶźČ«·“Ó¦µĆµ½µÄµē×ÓŹżĪŖ2NA
B£®1 L 2 mol”¤L-1 MgCl2ČÜŅŗÖŠŗ¬ÓŠµÄCl- ŹżĪŖ2NA
C. ±ź×¼×“æöĻĀ£¬11.2 LĀČĘųÓė×ćĮæNaOHČÜŅŗ·“Ó¦×ŖŅʵĵē×ÓŹżĪŖ0.5NA
D. ±ź×¼×“æöĻĀ£¬2.24 L CO2Óė2.24 L H2OÖŠĖłŗ¬Ō×ÓŹż¾łĪŖ0.3NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĪŽÉ«ČÜŅŗÖŠŗ¬ÓŠK£«”¢Cl£”¢OH£”¢SO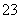 ”¢SO
”¢SO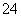 £¬ĪŖ¼ģŃéČÜŅŗÖŠĖłŗ¬µÄijŠ©ŅõĄė×Ó£¬ĻŽÓƵďŌ¼ĮÓŠ£ŗŃĪĖį”¢ĻõĖį”¢ĻõĖįŅųČÜŅŗ”¢ĻõĖį±µČÜŅŗ”¢äåĖ®ŗĶ·ÓĢŖŹŌŅŗ”£¼ģŃéĘäÖŠOH£µÄŹµŃé·½·ØŹ”ĀŌ£¬¼ģŃéĘäĖūŅõĄė×ӵĹż³ĢČēĻĀĶ¼ĖłŹ¾”£
£¬ĪŖ¼ģŃéČÜŅŗÖŠĖłŗ¬µÄijŠ©ŅõĄė×Ó£¬ĻŽÓƵďŌ¼ĮÓŠ£ŗŃĪĖį”¢ĻõĖį”¢ĻõĖįŅųČÜŅŗ”¢ĻõĖį±µČÜŅŗ”¢äåĖ®ŗĶ·ÓĢŖŹŌŅŗ”£¼ģŃéĘäÖŠOH£µÄŹµŃé·½·ØŹ”ĀŌ£¬¼ģŃéĘäĖūŅõĄė×ӵĹż³ĢČēĻĀĶ¼ĖłŹ¾”£
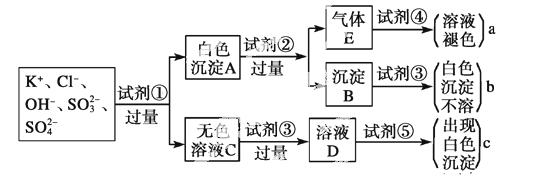
(1)Ķ¼ÖŠŹŌ¼Į¢Ł”«¢ŻČÜÖŹµÄ»ÆѧŹ½·Ö±šŹĒ
¢Ł________£¬¢Ś________£¬¢Ū________£¬¢Ü__________£¬
¢Ż__________”£
(2)Ķ¼ÖŠĻÖĻóa”¢b”¢c±ķĆ÷¼ģŃé³öµÄĄė×Ó·Ö±šŹĒ
a________Ӣb___ _____Ӣc________ӣ
_____Ӣc________ӣ
(3)°×É«³ĮµķA¼ÓŹŌ¼Į¢Ś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_________________________________
________________________________________________________________________ӣ
(4)ĪŽÉ«ČÜŅŗC¼ÓŹŌ¼Į¢ŪµÄÖ÷ŅŖÄæµÄŹĒ___________________________ __________”£
__________ӣ
(5)°×É«³ĮµķAČō¼ÓŹŌ¼Į¢Ū¶ų²»¼ÓŹŌ¼Į¢Ś£¬¶ŌŹµŃéµÄÓ°ĻģŹĒ____________________”£
(6)ĘųĢåEĶØČėŹŌ¼Į¢Ü·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ_______________________________
________________________________________________________________________ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com