

| ”÷n |
| V”÷t |
| 0.4mol |
| 1L2min |
| ”÷n |
| V”÷t |
| 1.2mol |
| 1L2min |
| ”÷n |
| V”÷t |
| 0.8mol |
| 1L2min |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 2NH3£¬2minŗó²āµĆN2µÄĪļÖŹµÄĮæĪŖ0.6mol£¬Ōņ£ŗ
2NH3£¬2minŗó²āµĆN2µÄĪļÖŹµÄĮæĪŖ0.6mol£¬Ōņ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
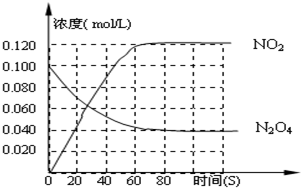 ŌŚŅ»Ģå»żĪŖ1LµÄČŻĘ÷ÖŠ£¬ĶØČėŅ»¶ØĮæµÄN2O4£¬ŌŚ100”ꏱ·¢ÉśČēĻĀ·“Ó¦£¬N2O4
ŌŚŅ»Ģå»żĪŖ1LµÄČŻĘ÷ÖŠ£¬ĶØČėŅ»¶ØĮæµÄN2O4£¬ŌŚ100”ꏱ·¢ÉśČēĻĀ·“Ó¦£¬N2O4  2NO2-Q£ØQ£¾0£©£¬ĘäN2O4 ŗĶNO2 ÅØ¶Č±ä»ÆČēĶ¼ČēŹ¾£®
2NO2-Q£ØQ£¾0£©£¬ĘäN2O4 ŗĶNO2 ÅØ¶Č±ä»ÆČēĶ¼ČēŹ¾£®| [c(NO2)]2 |
| c(N2O4) |
| [c(NO2)]2 |
| c(N2O4) |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÉĻŗ£ŹŠøßČżÉĻŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
ŌŚŅ»Ģå»żĪŖ1LµÄČŻĘ÷ÖŠ£¬ĶØČėŅ»¶ØĮæµÄN2O4
£¬ŌŚ100”ꏱ·¢ÉśČēĻĀ·“Ó¦£¬N2O4 2NO2ØCQ£ØQ>0£©£¬ĘäN2O4 ŗĶNO2 ÅØ¶Č±ä»ÆČēĶ¼ČēŹ¾”£
2NO2ØCQ£ØQ>0£©£¬ĘäN2O4 ŗĶNO2 ÅØ¶Č±ä»ÆČēĶ¼ČēŹ¾”£

£Ø1£© ÉĻŹö·“Ó¦µÄĘ½ŗā³£Źż±ķ“ļŹ½ĪŖ______________£¬ÉżøßĪĀ¶ČKÖµ_______£ØĢī”°Ōö“ó”±”¢”°¼õŠ””±»ņ”°²»±ä”±£©
£Ø2£© ŌŚ0-60sÕā¶ĪŹ±¼äÄŚ£¬ĖÄŃõ»Æ¶žµŖµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ________mol/L£®s
£Ø3£©120”ꏱ£¬ŌŚĻąĶ¬µÄČŻĘ÷ÖŠ·¢ÉśÉĻŹö·“Ó¦£¬ČŻĘ÷ÄŚø÷ĪļÖŹµÄĪļÖŹµÄĮæ±ä»ÆČēĶ¼£ŗ
 |
¢ŁøĆĪĀ¶ČŹ±£¬·“Ó¦“¦ÓŚĘ½ŗāדĢ¬µÄŹ±¼äŹĒ____________£¬C1µÄŹżÖµ_____0£®04£ØĢī”°“óÓŚ”±”¢”°Š”ÓŚ”±»ņ”°µČÓŚ”±”£
¢Ś·“Ó¦ŌŚ60-80s¼äĘ½ŗāĻņÄę·“Ó¦·½ĻņŅĘ¶Æ£¬æÉÄܵÄŌŅņŹĒ£Ø £©
£ØA£© Ź¹ÓĆ“ß»Æ¼Į £ØB£© ¼õÉŁN2O4µÄÅضČ
£ØC£©¼õŠ”ĢåĻµŃ¹Ēæ £ØD£© Ōö¼ÓNO2µÄÅضČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2009-2010ѧğŗžÄĻŹ”ĻęĪ÷ÖŻ·ļ»ĖĻŲ»ŖöĪ֊ѧøßŅ»£ØĻĀ£©ĘŚÖŠ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
 2NH3£¬2minŗó²āµĆN2µÄĪļÖŹµÄĮæĪŖ0.6mol£¬Ōņ£ŗ
2NH3£¬2minŗó²āµĆN2µÄĪļÖŹµÄĮæĪŖ0.6mol£¬Ōņ£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com