
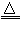 2MgO+CO2Γϋ+H2OΓϋ Θ®ΜρaMgCO3ΓΛbMg(OH)2
2MgO+CO2Γϋ+H2OΓϋ Θ®ΜρaMgCO3ΓΛbMg(OH)2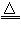 (a+b)MgO+aCO2Γϋ+bH2OΓϋ Θ©
(a+b)MgO+aCO2Γϋ+bH2OΓϋ Θ©

 »ΪΡή≤βΩΊ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
»ΪΡή≤βΩΊ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
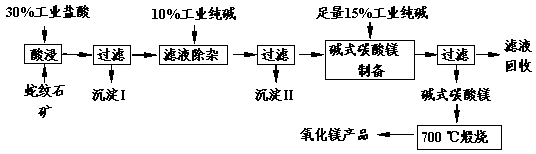
Θ®16Ζ÷Θ©…ΏΈΤ ·“ρΤδΜ®ΈΤΥΤ…ΏΤΛΕχΒΟΟϊΘ§Ρ≥ΒΊ…ΏΈΤ ·¥σ‘ΦΚ§MgO38%Θ§ΝμΆβ≥ΐΝΥΚ§SiO2ΆβΘ§ΜΙΚ§”–CaOΓΔFe2O3ΓΔAl2O3Β»―θΜ·ΈοΘ§”……ΏΈΤ ·Ωσ÷Τ±ΗMgOΒΡΙΛ“’Νς≥Χ»γœ¬ΓΘ
Θ®1Θ©–¥≥ωΥαΫΰΙΐ≥Χ÷–ΒΡ“ΜΗωάκΉ”ΖΫ≥Χ Ϋ ΓΘ»τ‘Ύ Β―ι “Ϋχ––ΥαΫΰΙΐ≥ΧΘ§–η“ΣΒΡ“«Τς”–…’±≠ΓΔ ΓΘ
Θ®2Θ©≥ΝΒμIΒΡ≥…Ζ÷ « Θ®–¥Μ·―ß ΫΘ©ΓΘ–¥≥ωΥϋΒΡ“ΜΗω”ΟΆΨ ΓΘ
Θ®3Θ©≥ΝΒμIIΒΡ≥…Ζ÷ « ΓΘ
Θ®4Θ©¥”¬Υ“Κ÷–ΡήΜΊ ’ΒΡΈο÷ ”– ΓΘ
Θ®5Θ©–¥≥ωΦν ΫΧΦΥαΟΨ700 Γφλ―…’≤ζ…ζ―θΜ·ΟΨΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2011-2012ΫλΙψΕΪ Γ…ΊΙΊ –ΗΏ»ΐœ¬―ßΤΎΒΎΕΰ¥ΈΒς―–ΩΦ ‘Μ·―ß ‘ΨμΘ®¥χΫβΈωΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®16Ζ÷Θ©…ΏΈΤ ·“ρΤδΜ®ΈΤΥΤ…ΏΤΛΕχΒΟΟϊΘ§Ρ≥ΒΊ…ΏΈΤ ·¥σ‘ΦΚ§MgO38%Θ§ΝμΆβ≥ΐΝΥΚ§SiO2ΆβΘ§ΜΙΚ§”–CaOΓΔFe2O3ΓΔAl2O3Β»―θΜ·ΈοΘ§”……ΏΈΤ ·Ωσ÷Τ±ΗMgOΒΡΙΛ“’Νς≥Χ»γœ¬ΓΘ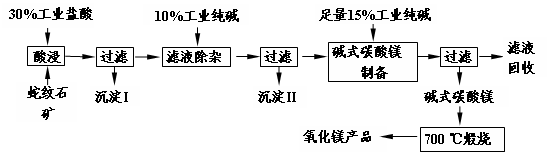
Θ®1Θ©–¥≥ωΥαΫΰΙΐ≥Χ÷–ΒΡ“ΜΗωάκΉ”ΖΫ≥Χ Ϋ ΓΘ»τ‘Ύ Β―ι “Ϋχ––ΥαΫΰΙΐ≥ΧΘ§–η“ΣΒΡ“«Τς”–…’±≠ΓΔ ΓΘ
Θ®2Θ©≥ΝΒμIΒΡ≥…Ζ÷ « Θ®–¥Μ·―ß ΫΘ©ΓΘ–¥≥ωΥϋΒΡ“ΜΗω”ΟΆΨ ΓΘ
Θ®3Θ©≥ΝΒμIIΒΡ≥…Ζ÷ « ΓΘ
Θ®4Θ©¥”¬Υ“Κ÷–ΡήΜΊ ’ΒΡΈο÷ ”– ΓΘ
Θ®5Θ©–¥≥ωΦν ΫΧΦΥαΟΨ700 Γφλ―…’≤ζ…ζ―θΜ·ΟΨΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2011-2011-2012―ßΡξΙψΕΪ Γ…ΊΙΊ –ΗΏ»ΐœ¬―ßΤΎΒΎΕΰ¥ΈΒς―–ΩΦ ‘Μ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚΧνΩ’Χβ
Θ®16Ζ÷Θ©…ΏΈΤ ·“ρΤδΜ®ΈΤΥΤ…ΏΤΛΕχΒΟΟϊΘ§Ρ≥ΒΊ…ΏΈΤ ·¥σ‘ΦΚ§MgO38%Θ§ΝμΆβ≥ΐΝΥΚ§SiO2ΆβΘ§ΜΙΚ§”–CaOΓΔFe2O3ΓΔAl2O3Β»―θΜ·ΈοΘ§”……ΏΈΤ ·Ωσ÷Τ±ΗMgOΒΡΙΛ“’Νς≥Χ»γœ¬ΓΘ

Θ®1Θ©–¥≥ωΥαΫΰΙΐ≥Χ÷–ΒΡ“ΜΗωάκΉ”ΖΫ≥Χ Ϋ ΓΘ»τ‘Ύ Β―ι “Ϋχ––ΥαΫΰΙΐ≥ΧΘ§–η“ΣΒΡ“«Τς”–…’±≠ΓΔ ΓΘ
Θ®2Θ©≥ΝΒμIΒΡ≥…Ζ÷ « Θ®–¥Μ·―ß ΫΘ©ΓΘ–¥≥ωΥϋΒΡ“ΜΗω”ΟΆΨ ΓΘ
Θ®3Θ©≥ΝΒμIIΒΡ≥…Ζ÷ « ΓΘ
Θ®4Θ©¥”¬Υ“Κ÷–ΡήΜΊ ’ΒΡΈο÷ ”– ΓΘ
Θ®5Θ©–¥≥ωΦν ΫΧΦΥαΟΨ700 Γφλ―…’≤ζ…ζ―θΜ·ΟΨΒΡΜ·―ßΖΫ≥Χ Ϋ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
…ΏΈΤ ·“ρΤδΜ®ΈΤΥΤ…ΏΤΛΕχΒΟΟϊΘ§Ρ≥ΒΊ…ΏΈΤ ·¥σ‘ΦΚ§MgO38%Θ§ΝμΆβ≥ΐΝΥΚ§SiO2ΆβΘ§ΜΙΚ§”–CaOΓΔFe2O3ΓΔAl2O3Β»―θΜ·ΈοΘ§”……ΏΈΤ ·Ωσ÷Τ±ΗMgOΒΡΙΛ“’Νς≥Χ»γœ¬ΓΘ

Θ®1Θ©–¥≥ωΥαΫΰΙΐ≥Χ÷–ΒΡ“ΜΗωάκΉ”ΖΫ≥Χ Ϋ
»τ‘Ύ Β―ι “Ϋχ––ΥαΫΰΙΐ≥ΧΘ§–η“ΣΒΡ“«Τς”–…’±≠ΓΔ ΓΘ
Θ®2Θ©≥ΝΒμIΒΡ≥…Ζ÷ « Θ®–¥Μ·―ß ΫΘ©ΓΘ–¥≥ωΥϋΒΡ“ΜΗω”ΟΆΨ ΓΘ
Θ®3Θ©≥ΝΒμIIΒΡ≥…Ζ÷ « ΓΔ ΓΔ ΓΘ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΑΌΕ»÷¬–≈ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com