
 ��֪�Ȼ�ѧ����ʽH+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol��
��֪�Ȼ�ѧ����ʽH+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol������ ��1�������Ȼ�ѧ����ʽ�õ���Ӧ����1molˮ�ų����������ٸ��ݱ����ݼ������ߵ��¶ȣ�
��2��A����ͼʾ�۲���ʼ�¶ȼ�Ϊʵ��ʱ�����¶ȣ�
B������ͼʾ������Һ�¶ȱ仯���з�����
C����������������Һ��������Һ��Ӧ����ʽ���м��㣻
D������һ����Ӧ���ó��˽��ۣ�
��3��A��ˮ����ˮ���ӹ��ɵģ�ˮ����������������Ԫ����ɣ�������������Ԫ�أ�����Ҳ��������Ԫ�أ�
B����ˮ��ȡ����Դ������ˮ�ڸ�����Ҳ���Էֽ⣬�跨��̫����۽�����������ʹˮ�ֽ����������
C���������ܼӿ컯ѧ��Ӧ���ʣ���ˮ�ķֽ������ȷ�Ӧ��
D��Ѱ������Ļ�ѧ���ʣ��ı�ˮ�ֽ�Ļ�ѧ��Ӧ���ʣ����ڿ���������Դ���Էֽ�ˮ��ȡ������
��4�����ø�˹���ɿ��Ը�����֪�ķ�Ӧ���Ȼ�ѧ����ʽ��Ӧ2CO+SO2=S+2CO2���ʱ䣬����д�Ȼ�ѧ����ʽ��
��� �⣺��1��H+��aq��+OH-��aq���TH2O��l����H=-57.3kJ/mol��0.25L 0.10mol/L��һԪǿ���ǿ���к�����0.025molH2O����Ӧ����0.025mol��57.3kJ/mol=1.4325KJ�����кͺ���Һ���Ϊ0.5L���кͺ����Һ�ı�����Ϊ4.2��10-3kJ/��g•�棩�����ܶ�Ϊ1.0g/mL������Q=-C��T2-T1�����õ�-1.4325KJ=-4.2��10-3kJ/��g•�棩��500ml��1.0g/mL����T����T=0.68��C
�ʴ�Ϊ��0.68��
��2��A����ʵ�鿪ʼ�¶���21�棬��A����
B����ͼʾ���Կ����÷�Ӧ���̷ų�������������ѧ�ܿ���ת��Ϊ���ܣ���B��ȷ��
C��ǡ�÷�Ӧʱ�μӷ�Ӧ��������Һ�������30mL����V1+V2=50Ml��֪�����ĵ�����������Һ�����Ϊ20mL��
��ǡ�÷�Ӧʱ����������Һ�����ʵ����ʵ�����n��
HCl+NaOH=NaCl+H2O
1 1
1.0mol•L-1��0.03L n
��n=1.0mol•L-1��0.03L=0.03mol������Ũ���ǣ�$\frac{0.03mol}{0.02L}$=1.5mol/L����C����
D��ֻ�Ǹ÷�Ӧ���ȣ�������ˮ���ɵķ�Ӧ��һ��������D����
�ʴ�Ϊ��B��
��3��A�����������������ֵ��ʣ��������п�ȼ�ԣ��ɳ�Ϊ����Դ������������ȼ�գ�ֻ����ȼ�ԣ�ˮ������Ԫ�غ���Ԫ����ɣ�Ԫ�ز���ͬ�ڵ��ʣ����ԣ�����ˮ����������ǿ���ȼ�յ����ʣ�˵������ˮ�ڲ��ֽ������£�������������ʣ������������ʣ���û��������������ˮ�Ͳ����Ϊ����Դ����˿��о���ˮ���ֽ������£�ʹ���Ϊ������Դ����A����
B��̫������һ��������Դ��ˮ�ڸ����¿��Էֽ⣬�跨��̫����۽����������£�ʹˮ�ֽ�����������������������п�ȼ�ԣ�����Ϊ����Դ����B��ȷ��
C��ʹ�ô��������Լӿ컯ѧ��Ӧ���ʣ���ˮ�ķֽ������ȷ�Ӧ����C����
D��ˮ���Էֽ�����H2���������п�ȼ�ԣ�ȼ�շ��ȣ����ֽ�ˮ�����������������������Ѱ������Ļ�ѧ���ʣ��������ڸ��������·ֽ�ˮ�����ͳɱ������ڿ���������Դ����D��ȷ��
�ʴ�Ϊ��AC��
��4����C��s��+O2��g���TCO2��g����H1=-393.5kJ•mol-1
��CO2��g��+C��s���T2CO��g����H2=+172.5kJ•mol-1
��S��s��+O2��g���TSO2��g����H3=-296.0kJ•mol-1
���ݸ�˹���ɣ���-��-�۵õ�2CO��g��+SO2��g���TS��s��+2CO2��g����H=��H1-��H2-��H3=-270 kJ•mol-1��
�ʴ�Ϊ��2CO��g��+SO2��g���TS��s��+2CO2��g����H=-270 kJ•mol-1��
���� ���⿼���к��Ȳⶨ�����ͼ���Ӧ�ã��Ȼ�ѧ����ʽ��˹���ɵļ����������Դ�����жϣ�ע��ѧϰ������Ԫ���뵥�ʵĹ�ϵ�����ջ����ǹؼ�����Ŀ�ϼ�


 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ù��˵ķ�������CaCO3��CaCl2��Һ�Ļ���� | |
| B�� | �ýᾧ���ᴿNaCl��KNO3������е�KNO3 | |
| C�� | ���������Ҵ���ˮ�Ļ���� | |
| D�� | �ü��ȷ��������Ȼ�淋Ļ�����ʾ�������������Ȼ�������ֽ⣮��ȴ�����Ƕ����������ɹ��壩 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ٺ͢� | B�� | �ٺ͢� | C�� | �ں͢� | D�� | ����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
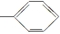 ��-CHO ��-H��
��-CHO ��-H��| A�� | 4�� | B�� | 5�� | C�� | 6�� | D�� | 7�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ϊ����ȡ�������������õĽ������ɽ�����з��� | |
| B�� | ģ��һ��ɷ�Ϊ����ģ�ͺ�˼άģ�� | |
| C�� | ijͬѧ�о�SO2���ʵij����Ƿ��ࣨԤ��SO2�Ļ�ѧ���ʣ����۲죨�ó�SO2���������ʣ���ʵ����Ƚϲ��ó����� | |
| D�� | ��ѧʵ���ܽ����ѧѧ�Ƶ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1molH2����Ϊ2g | B�� | H2O��Ħ������Ϊ18g | ||
| C�� | �����44g CO2�����Ϊ22.4L | D�� | 9.8g H2SO4��1mol��H2SO4���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
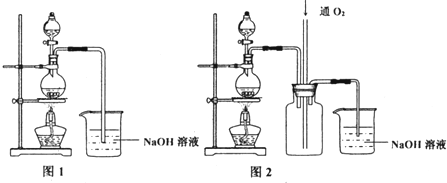
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 25��ʱ������ƽ�ⳣ����
25��ʱ������ƽ�ⳣ����| ��ѧ�� | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ�� | 1.8��10-5 | K14.3��10-7 K25.6��10-11 | 3.0��10-8 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com