
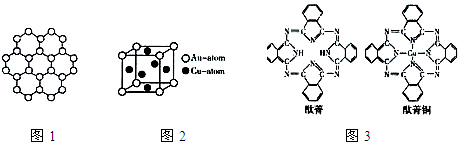
| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
| 1 |
| 8 |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
 Čšµä»Ź¼ŅæĘѧŌŗŠū²¼£¬½«2010ÄźÅµ±“¶ūĪļĄķѧ½±ŹŚÓč Ó¢¹śĀü³¹Ė¹ĢŲ“óѧæĘѧ¼Ņ°²µĀĮŅ?ŗ£Ä·ŗĶæµĖ¹Ģ¹¶”?ŵĪÖŠ¤Āå·ņ£¬ŅŌ±ķÕĆĖūĆĒŌŚŹÆÄ«Ļ©²ÄĮĻ·½ĆęµÄ×æŌ½ŃŠ¾æ£®ŹÆÄ«Ļ©ŹĒÓÉĢ¼Ō×Ó¹¹³ÉµÄµ„²ćʬד½į¹¹µÄŠĀ²ÄĮĻ£®¾ßÓŠŌ×Ó¼¶µÄŗń¶Č”¢ÓÅŅģµÄµēѧŠŌÄÜ”¢³öÉ«µÄ»ÆѧĪČ¶ØŠŌŗĶČČĮ¦Ń§ĪČ¶ØŠŌ£®ÖʱøŹÆÄ«Ļ©·½·ØÓŠŹÆÄ«°žĄė·Ø”¢»ÆѧĘųĻą³Į»ż·ØµČ£®ŹÆÄ«Ļ©µÄĒņ¹÷Ä£ŠĶ¼°·Ö×Ó½į¹¹ČēĶ¼1ĖłŹ¾£®
Čšµä»Ź¼ŅæĘѧŌŗŠū²¼£¬½«2010ÄźÅµ±“¶ūĪļĄķѧ½±ŹŚÓč Ó¢¹śĀü³¹Ė¹ĢŲ“óѧæĘѧ¼Ņ°²µĀĮŅ?ŗ£Ä·ŗĶæµĖ¹Ģ¹¶”?ŵĪÖŠ¤Āå·ņ£¬ŅŌ±ķÕĆĖūĆĒŌŚŹÆÄ«Ļ©²ÄĮĻ·½ĆęµÄ×æŌ½ŃŠ¾æ£®ŹÆÄ«Ļ©ŹĒÓÉĢ¼Ō×Ó¹¹³ÉµÄµ„²ćʬד½į¹¹µÄŠĀ²ÄĮĻ£®¾ßÓŠŌ×Ó¼¶µÄŗń¶Č”¢ÓÅŅģµÄµēѧŠŌÄÜ”¢³öÉ«µÄ»ÆѧĪČ¶ØŠŌŗĶČČĮ¦Ń§ĪČ¶ØŠŌ£®ÖʱøŹÆÄ«Ļ©·½·ØÓŠŹÆÄ«°žĄė·Ø”¢»ÆѧĘųĻą³Į»ż·ØµČ£®ŹÆÄ«Ļ©µÄĒņ¹÷Ä£ŠĶ¼°·Ö×Ó½į¹¹ČēĶ¼1ĖłŹ¾£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 £Ø2012?³¤ÄžĒų¶žÄ££©¶ĢÖÜĘŚÖŠ³£¼ū½šŹōX”¢ŹÆÄ«ŗĶ¶žŃõ»ÆīŃ£ØTiO2£©°“±ČĄż»ģŗĻ£¬øßĪĀĻĀ·“Ó¦µĆµ½µÄ»ÆŗĻĪļ¾łÓÉĮ½ÖÖŌŖĖŲ×é³É£¬ĒŅ¶¼ŹĒŠĀŠĶĢՓɲÄĮĻ£ØŌŚ»š¼żŗĶµ¼µÆÉĻÓŠÖŲŅŖÓ¦ÓĆ£©£®øł¾ŻĢāŅāĶź³ÉĻĀĮŠĢīæÕ£ŗ
£Ø2012?³¤ÄžĒų¶žÄ££©¶ĢÖÜĘŚÖŠ³£¼ū½šŹōX”¢ŹÆÄ«ŗĶ¶žŃõ»ÆīŃ£ØTiO2£©°“±ČĄż»ģŗĻ£¬øßĪĀĻĀ·“Ó¦µĆµ½µÄ»ÆŗĻĪļ¾łÓÉĮ½ÖÖŌŖĖŲ×é³É£¬ĒŅ¶¼ŹĒŠĀŠĶĢՓɲÄĮĻ£ØŌŚ»š¼żŗĶµ¼µÆÉĻÓŠÖŲŅŖÓ¦ÓĆ£©£®øł¾ŻĢāŅāĶź³ÉĻĀĮŠĢīæÕ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 2010Äź10ŌĀ5ČÕ±±¾©Ź±¼ä17Ź±45·ÖČšµä»Ź¼ŅæĘѧŌŗŠū²¼£¬½«2010ÄźÅµ±“¶ūĪļĄķѧ½±ŹŚÓčÓ¢¹śĀü³¹Ė¹ĢŲ“óѧæĘѧ¼Ņ°²µĀĮŅ?ŗ£Ä·ŗĶæµĖ¹Ģ¹¶”?ŵĪÖŠ¤Āå·ņ£®¹²ŹĀ¶ąÄźµÄ¶žČĖŅņ”°Ķ»ĘĘŠŌµŲ”±ÓĆĖŗĮѵķ½·Ø³É¹¦»ńµĆ³¬±”²ÄĮĻŹÆÄ«Ļ©£ØČēĶ¼£©¶ų»ń½±£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©
2010Äź10ŌĀ5ČÕ±±¾©Ź±¼ä17Ź±45·ÖČšµä»Ź¼ŅæĘѧŌŗŠū²¼£¬½«2010ÄźÅµ±“¶ūĪļĄķѧ½±ŹŚÓčÓ¢¹śĀü³¹Ė¹ĢŲ“óѧæĘѧ¼Ņ°²µĀĮŅ?ŗ£Ä·ŗĶæµĖ¹Ģ¹¶”?ŵĪÖŠ¤Āå·ņ£®¹²ŹĀ¶ąÄźµÄ¶žČĖŅņ”°Ķ»ĘĘŠŌµŲ”±ÓĆĖŗĮѵķ½·Ø³É¹¦»ńµĆ³¬±”²ÄĮĻŹÆÄ«Ļ©£ØČēĶ¼£©¶ų»ń½±£®ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø””””£©²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
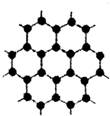 2010Äź10ŌĀ5ČÕ17Ź±45·Ö£¬Čšµä»Ź¼ŅæĘѧŌŗŠū²¼£¬½«2010ÄźÅµ±“¶ūĪļĄķѧ½±ŹŚÓčÓ¢¹śĀü³¹Ė¹ĢŲ“óѧæĘѧ¼Ņ°²µĀĮŅ?ŗ£Ä·ŗĶæµĖ¹Ģ¹¶”?ŵĪÖŠ¤Āå·ņ£¬ŅŌ±ķÕĆĖūĆĒŌŚŹÆÄ«Ļ©²ÄĮĻ·½ĆęµÄ×æŌ½ŃŠ¾æ£®ŹÆÄ«Ļ©ŹĒÄæĒ°æĘ¼¼ŃŠ¾æµÄČČµć£¬æÉæ“×÷½«ŹÆÄ«µÄ²ćד½į¹¹Ņ»²ćŅ»²ćµÄ°žæŖµĆµ½µÄµ„²ćĢ¼Ō×ӣؽį¹¹ČēĶ¼ĖłŹ¾£©£®½«ĒāĘų¼ÓČėµ½ŹÆÄ«Ļ©ÖŠæŖ·¢³öŅ»ÖÖ¾ßÓŠĶ»ĘĘŠŌµÄŠĀ²ÄĮĻŹÆÄ«Ķ飬ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ£Ø””””£©
2010Äź10ŌĀ5ČÕ17Ź±45·Ö£¬Čšµä»Ź¼ŅæĘѧŌŗŠū²¼£¬½«2010ÄźÅµ±“¶ūĪļĄķѧ½±ŹŚÓčÓ¢¹śĀü³¹Ė¹ĢŲ“óѧæĘѧ¼Ņ°²µĀĮŅ?ŗ£Ä·ŗĶæµĖ¹Ģ¹¶”?ŵĪÖŠ¤Āå·ņ£¬ŅŌ±ķÕĆĖūĆĒŌŚŹÆÄ«Ļ©²ÄĮĻ·½ĆęµÄ×æŌ½ŃŠ¾æ£®ŹÆÄ«Ļ©ŹĒÄæĒ°æĘ¼¼ŃŠ¾æµÄČČµć£¬æÉæ“×÷½«ŹÆÄ«µÄ²ćד½į¹¹Ņ»²ćŅ»²ćµÄ°žæŖµĆµ½µÄµ„²ćĢ¼Ō×ӣؽį¹¹ČēĶ¼ĖłŹ¾£©£®½«ĒāĘų¼ÓČėµ½ŹÆÄ«Ļ©ÖŠæŖ·¢³öŅ»ÖÖ¾ßÓŠĶ»ĘĘŠŌµÄŠĀ²ÄĮĻŹÆÄ«Ķ飬ĻĀĮŠĖµ·ØÖŠÕżČ·µÄŹĒ£Ø””””£©| A”¢ŹÆÄ«Ļ©ŹĒøß·Ö×Ó»ÆŗĻĪļ | B”¢ŹÆÄ«Ļ©ÓėŹÆÄ«Ķ黄ĪŖĶ¬ĖŲŅģŠĪĢå | C”¢Ņ»¶ØĢõ¼žĻĀŹÆÄ«Ļ©æÉÓėH2·¢Éś¼Ó³É·“Ó¦ | D”¢øł¾Ż½į¹¹Ź¾ŅāĶ¼æÉÖŖ£¬ŹÆÄ«Ļ©²»Äܵ¼µē |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com