
| ||
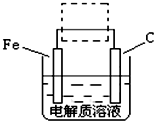 ��FeSO4?7H2O��һ��ˮ����ԭ��Ϊ
��FeSO4?7H2O��һ��ˮ����ԭ��Ϊ 3Zn��OH��2+2Fe��OH��3+4KOH
3Zn��OH��2+2Fe��OH��3+4KOH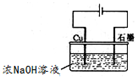 ��Һ��pHΪ
��Һ��pHΪ
| ||
 Fe��OH��3+3H+��
Fe��OH��3+3H+��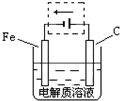



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ѧϰ�ܱ�����ѧ���˽̿α��һ��(����1)��2009��2010ѧ�ꡡ��7�ڡ��ܵ�163�� �˽̿α�� ���ͣ�022
ͭ�У�1�ۺͣ�2�ۣ�ͭ����Ͻ����ճ�������Ӧ�ù㷺������������Ϣ�ش�������⣮
(1)��ҵұ��ͭ��ԭ����Ҫ�ǣ�
(��)2Cu2S(����ͭ)��3O2![]() 2Cu2O��2SO2
2Cu2O��2SO2
(��)2Cu2O(������ͭ)��Cu2S![]() 6Cu��SO2����
6Cu��SO2����
�ٹ���������ͭ������ͭ���жϣ���ȷ����

�������Ӧ(��)�ͷ�Ӧ(��)�����Ķ�������������ȣ���ת�Ƶĵ�����֮��Ϊ________
A��1��1
B��2��1
C��1��2
D��3��1
(2)���������ڡ�������������н������ҹ�������ʱ�ڷ�����ʪ��ұ�������������������Ϊͭ�����京����ͭ�Ŀ����Ի������ˮ��Һ������Ӧ����ͭ�����磬����ͭ��Һ������Ӧ��CuSO4��Fe![]() Cu��FeSO4�������йظ÷�Ӧ���жϣ��������û���Ӧ����������������ͭ����������������ԣ�FeSO4��CuSO4���ݻ�ԭ�ԣ�Fe��Cu�����в���ȷ����________��
Cu��FeSO4�������йظ÷�Ӧ���жϣ��������û���Ӧ����������������ͭ����������������ԣ�FeSO4��CuSO4���ݻ�ԭ�ԣ�Fe��Cu�����в���ȷ����________��
A���٢�
B���ڢ�
C���ۢ�
D���ڢ�
(3)�ִ�ұͭ���������Ȼ�ԭ������H2��CuO![]() Cu��H2O��2Al��3CuO
Cu��H2O��2Al��3CuO![]() 3Cu��Al2O3��CO��CuO
3Cu��Al2O3��CO��CuO![]() Cu��CO2������ȡ�������Ļ�ԭ����μӷ�Ӧ�Ļ�ԭ��H2��Al��CO��������Ϊ________��
Cu��CO2������ȡ�������Ļ�ԭ����μӷ�Ӧ�Ļ�ԭ��H2��Al��CO��������Ϊ________��
A��1��1��1
B��1��3��1
C��1��9��14
D��3��2��3
(4)ͭ�����治�ƣ��ڳ�ʪ�Ŀ����л���ͭ�⣬��Ҫ�Ļ�ѧ��ӦΪ2Cu��CO2��O2��H2O![]() Cu2(OH)2CO3(ͭ��)���÷�Ӧ���ڻ��Ϸ�Ӧ���жϵ�������________���÷�Ӧ��������ԭ��Ӧ���жϵ�������________������������________����ԭ������________���жϵ����ݷֱ���________��
Cu2(OH)2CO3(ͭ��)���÷�Ӧ���ڻ��Ϸ�Ӧ���жϵ�������________���÷�Ӧ��������ԭ��Ӧ���жϵ�������________������������________����ԭ������________���жϵ����ݷֱ���________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com