



 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ʡ����һ��2010��2011ѧ���һ��ѧ�ڵ�һѧ�ο��Ի�ѧ���� ���ͣ�013
|
��Ӧ��CO(g)��H2O(g)��CO2(g)��H2(g)�������仯��ͼ��ʾ���йظ÷�Ӧ��˵����ȷ����
| |
| [����] | |
A�� |
�÷�ӦΪ���ȷ�Ӧ |
B�� |
1 mol��CO2(g)��1 mol��H2(g)��Ӧ����1 mol��CO(g)��1 mol��H2O(g)Ҫ�ų�41 kJ���� |
C�� |
��Ӧ���Ȼ�ѧ����ʽ�ǣ�CO(g)��H2O(g)��CO2(g)��H2(g)��H����41 kJ/mol |
D�� |
CO(g)��H2O(g)���������������CO2(g)��H2(g)����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����ݱ����ص��ĸ���ѧ�߶�4���¿���ѧ������������ ���ͣ���ѡ��
CO(g)��H2O(g)��Ӧ���̵������仯����ͼ��ʾ���й����߷�Ӧ˵����ȷ����(����)
| A���÷�ӦΪ���ȷ�Ӧ |
| B��CO(g)��H2O(g)���������������CO2(g)��H2(g)��������� |
| C����Ӧ���Ȼ�ѧ����ʽ��CO(g)��H2O(g)===CO2(g)��H2(g)����H����41kJ��mol��1 |
| D��1mol CO2(g)��1mol H2(g)��Ӧ����1mol CO(g)��H2O(g)Ҫ�ų�41kJ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�����и߶���ѧ����ĩ���Ի�ѧ�Ծ���ѡ�ޣ��������棩 ���ͣ������
��ѧ��Ӧ�м������ʱ仯�����������仯���ͷŻ����������ǻ�ѧ��Ӧ�������仯����Ҫ��ʽ֮һ����֪C(ʯī)��H2(g)ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
�� C(ʯī)+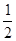 O2(g)��CO(g)
O2(g)��CO(g) 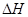 =-111.0 KJ��mol-1
=-111.0 KJ��mol-1
�� H2(g)+
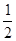 O2(g) ��H20(g)
O2(g) ��H20(g) 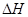 =-242.0 kJ��mol-1
=-242.0 kJ��mol-1
�� C(ʯī)+O2(g)��CO2(g)
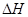 =-394.0 kJ��mol-1
=-394.0 kJ��mol-1
�����������⣺
��1����ѧ��Ӧ���������仯�ı���ԭ���Ƿ�Ӧ�������� �Ķ��Ѻ��γɡ�����������Ӧ���� (����ȡ����ȡ�)��Ӧ��
��2�����Ȼ�ѧ����ʽ�У���Ҫ������Ӧ�P�������״̬��ԭ���� ���ڢ��У�02�Ļ�ѧ��������1/2���DZ�ʾ (����ĸ)��
a�����Ӹ��� b�����ʵ��� c����������
��3����Ӧ2H20(g)��2H2(g)+02(g)��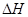 = KJ��mol-1��
= KJ��mol-1��
��4����C(���ʯ)+02(g)��C02(g)��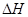 =-395.0 kJ��mol-1�����ȶ��ԣ����ʯ (�����������������)ʯī��
=-395.0 kJ��mol-1�����ȶ��ԣ����ʯ (�����������������)ʯī��
��5����֪�γ�H20(g)�е�2 mol H-O���ܷų�926.0 kJ���������γ�1 mol 02(g)�еĹ��ۼ��ܷų�498.0 kJ�������������1 mol H2(g)�е�H-H����Ҫ������ KJ��
��6����ҵ��������һ����Ҫ;������CO(g)��H2O(g)��Ӧ����C02(g)��H2(g)����÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�߶�4���¿���ѧ���������棩 ���ͣ�ѡ����
CO(g)��H2O(g)��Ӧ���̵������仯����ͼ��ʾ���й����߷�Ӧ˵����ȷ����(����)

A���÷�ӦΪ���ȷ�Ӧ
B��CO(g)��H2O(g)���������������CO2(g)��H2(g)���������
C����Ӧ���Ȼ�ѧ����ʽ��CO(g)��H2O(g)===CO2(g)��H2(g)����H����41kJ��mol��1
D��1mol CO2(g)��1mol H2(g)��Ӧ����1mol CO(g)��H2O(g)Ҫ�ų�41kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ѧ��Ӧ�м������ʱ仯�����������仯���ͷŻ����������ǻ�ѧ��Ӧ�������仯����Ҫ��ʽ֮һ����֪C(ʯī)��H2(g)ȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
�� C(ʯī)+![]() O2(g)��CO(g)
O2(g)��CO(g) ![]() =-111.0 KJ��mol-1
=-111.0 KJ��mol-1
�� H2(g)+ ![]() 02(g) ��H20(g)
02(g) ��H20(g) ![]() =-242.0 kJ��mol-1
=-242.0 kJ��mol-1
�� C(ʯī)+02(g)��CO2(g) ![]() =-394.0 kJ��mol-1
=-394.0 kJ��mol-1
�����������⣺
(1)��ѧ��Ӧ���������仯�ı���ԭ���Ƿ�Ӧ�������� �Ķ��Ѻ��γɡ�����������Ӧ���� (����ȡ����ȡ�)��Ӧ��
(2)���Ȼ�ѧ����ʽ�У���Ҫ������Ӧ�P�������״̬��ԭ����
���ڢ��У�02�Ļ�ѧ��������1/2���DZ�ʾ (����ĸ)��
a�����Ӹ��� b�����ʵ��� c����������
(3)��Ӧ2H20(g)��2H2(g)+02(g)��![]() = KJ��mol-1��
= KJ��mol-1��
(4)��C(���ʯ)+02(g)��C02(g)��![]() =-395.0 kJ��mol-1�����ȶ��ԣ����ʯ
=-395.0 kJ��mol-1�����ȶ��ԣ����ʯ
(�����������������)ʯī��
(5)��֪�γ�H20(g)�е�2 mol H-O���ܷų�926.0 kJ���������γ�1 mol 02(g)�еĹ��ۼ��ܷų�498.0 kJ�������������1 mol H2(g)�е�H-H����Ҫ������ KJ��
(6)��ҵ��������һ����Ҫ;������CO(g)��H2O(g)��Ӧ����C02(g)��H2(g)����÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com