
| NaOH��Һ��ʼ���� | NaOH��Һ�յ���� | ������� | |
| ��һ�� | 0.10mL | 18.60mL | 20.00mL |
| �ڶ��� | 0.40mL | 19.00mL | 20.00mL |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| 0.2000mol/L��18.55mL |
| 20.00mL |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |
| V(��)��c(��) |
| V(����) |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ�鲽�� | Ԥ���������� |
| ����1��ȡ����������Ʒ������������ˮ�� | ������ȫ�ܽ����ɫ������Һ |
| ����2�� | |
| ����3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ�еμ�FeCl3��ϡ��Һ�����Ʊ�Fe��OH��3���� |
| B��������ζ��������ƣ��÷�̪��ָʾ��������ƿ����Һ����ɫ��ɺ�ɫʱ���ﵽ�ζ��յ� |
| C�������£���pH��ֽ�ⶨŨ��Ϊ0.1 mol?L-1 NaClO��Һ��0.1 mol?L-1 CH3COONa��Һ��pH���Ƚ�HClO��CH3COOH������ǿ�� |
| D�������£���Mg��OH��2�м��뱥���Ȼ����Һ��ʹMg��OH��2�ܽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
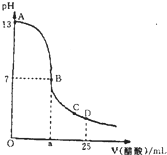 �����£���25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L CH3COOH��Һ��������ͼ������������Һ���ʱ������仯���й�����Ũ�ȱȽϻ�˵����ȷ���ǣ�������
�����£���25mL 0.1mol/L NaOH��Һ����μ���0.2mol/L CH3COOH��Һ��������ͼ������������Һ���ʱ������仯���й�����Ũ�ȱȽϻ�˵����ȷ���ǣ�������| A����A��B����һ�㣬��Һ��һ������c��Na+��+c��H+��=c��CH3COO-��+c��OH-�� |
| B����B��ʱ��a=12.5 |
| C����C�㣺c��CH3COO-����c��Na+����c��H+����c��OH-�� |
| D����D�㣺c��CH3COO-��=c��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���ڢޢ� |
| C���ۢܢ� | D���ܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��CH4 |
| B��CH3CH3 |
| C��C2H2 |
| D��C3H8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Ǧ���طŵ�ʱ�������ͳ��ʱ�ĸ���������������Ӧ |
| B���ñ���Na2CO3��Һ����BaSO4�������ɽ�BaSO4ת��ΪBaCO3 |
| C��һ���¶��£���ӦMg��1��+Cl2��g���TMgCl2��1���ġ�H��0����S��0 |
| D��pH=5��CH3COOH��Һ��pH=5��NH4C1��Һ�У�ˮ�ĵ���̶���ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com