
���й������������������ȷ���ǣ� ��
�ٷǽ��������ﲻһ���������������� �����������ﶼ���ڷǽ���������
�۽��������ﶼ���ڼ��������� �ܼ��������ﶼ���ڽ���������
�ݼ��������ﶼ����ˮ��Ӧ������Ӧ�ļ�
�������������������Ҳ����Ӧ�����κ�ˮ
����ˮ��Ӧ���ɺ������������һ��������������
������ᷴӦ��������һ������Ӧ
A���٢ڢۢޢ� B���ڢۢݢߢ� C���ڢۢݢޢ� D���ڢۢܢޢ�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C��Ϊ������Ԫ�أ����������ڱ��е�λ����ͼ��ʾ����֪��B��C��Ԫ��ԭ�ӵ�����������֮�͵���AԪ��ԭ�ӵ�������������2����B��C��Ԫ�صĺ˵����֮����AԪ��ԭ��������4������A��B��C�ֱ���(����)
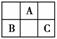
A��C��Al��P B��N��Si��S
C��O��P��Cl D��F��S��Ar
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ڱ�״���£���a L HCl(��)����1 000 gˮ�У��õ���������ܶ�Ϊb g��mL��1�������������ʵ���Ũ����(����)
A.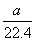 mol��L��1
mol��L��1
B.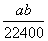 mol��L��1
mol��L��1
C.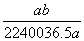 mol��L��1
mol��L��1
D.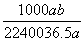 mol��L��1
mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
0.5L 1mol/L��FeCl3��Һ��0.2L 1 mol/L��KCl��Һ�У�Cl�������ʵ���Ũ��֮��Ϊ�� ��
A��15��2 B��1��1 C��3��1 D��1��3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ��������240mL 1mol/L������������Һ����ղ���ش��������⣺
(1) ������������� 1mol/L������������Һ
| Ӧ��ȡ�������ƹ��������/g | Ӧѡ������ƿ�Ĺ��/mL | ���ձ���������ƽ������ƿ����Ͳ������������ |
(2)����ʱ������ȷ�IJ���˳����(��ĸ��ʾ��ÿ����ĸֻ����һ��) ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B����������ƽȷ��ȡ������������ƹ��壬�����ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C��������ȴ����Һ�ز�����ע��һ����������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
(3)����A�У���ϴ��Һ����������ƿ����Ŀ���� ����Һע������ƿǰ��ָ������£�������Ϊ
��
(4)�����������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��?
��û�н���A���� ����������ˮʱ���������˿̶� ��������ʱ���ӿ̶���___________________��
(5)��ʵ������г������������δ�����
������ˮʱ���������˿̶� ��
������ƿ��ת����Һʱ(ʵ�鲽���)������Һ�ε�������ƿ����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ʼ��ÿ��ת������ͨ��һ����Ӧʵ�ֵ���
��Fe��Fe2O3��Fe(OH)3 �� Na��Na2O2��Na2CO3
�� S��SO3��H2SO4��MgSO4 �� C��CO��CO2��Na2CO3
A���٢� B���ۢ� C���ڢ� D���٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.195gп�ۼ��뵽20mL��0.100mol/LMO2+��Һ�У�ǡ����ȫ��Ӧ����ԭ��������ǣ� ��
A��M B�� C��
C��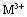 D��
D��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ����������(��Ҫ�ɷ�ΪAl2O3��Fe2O3��)��ȡAl2O3��ұ������ԭ�ϣ������ε�ⷨ��õĴ����к�һ�����Ľ����ƺ���������Щ���ʿɲ��ô�����������ȥ��������β��������������ڸֲĶ�����������������ͼ��ʾ��
(��֪��NaCl�۵�Ϊ801�棻AlCl3��181������)
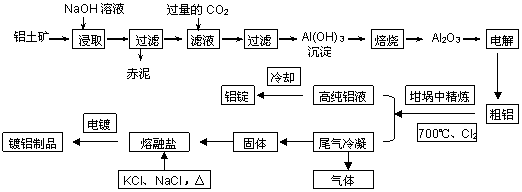
��1�������е���Ҫ�ɷ��� ����ѧʽ��������Һ��ͨ�����CO2��������Ӧ�����ӷ���ʽΪ ��
��2������ǰ������������������������ʯӢɰ����ֹ����ʱ���Ƿֱ����������û���Ӧ�����µ����ʣ���������������Ӧ�Ļ�ѧ����ʽΪ ��
��3����Cl2����ͨ�������еĴ������壬�����������ϸ�����ȥ�����ݵ���Ҫ�ɷֳ�Cl2�����______����̬����ճ���������ϣ�����������γɸ����������п϶�����________��
��4�����������У�������Ϊ�����������ε��Һ����Ԫ����Ҫ��AlCl4����ʽ���ڣ��������ĵ缫��ӦʽΪ_____________��
��5���ֲĶ�������ʴ���ܻ�����ǿ����ԭ����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���Ȼ�����S2Cl2����������ҵ���������������dzȻ�ɫ�ж����Һ�壬���ķ��ӽṹ��H2O2���ƣ��۵�Ϊ193 K���е�Ϊ411K����ˮ������Ӧ��������������ʹƷ����ɫ��S2Cl2���ɸ��������ͨ�����ڵ������Ƶá������й�˵�� ����ȷ����
����ȷ����
A��S2Cl2�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ
B���Ʊ�S2Cl2�ķ�Ӧ�ǻ��Ϸ�Ӧ������������ԭ��Ӧ
C��S2Cl2��ˮ��Ӧ�Ļ�ѧ����ʽΪ��2S2Cl2+2H2O=3S��+SO2��+4HCl
D��S2Br2��S2Cl2�ṹ���ƣ��۷е㣺S2Br2��S2Cl2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com