
��10�֣���֪��a��H+(aq) + OH-(aq) = H2O(l) ��H=-57.3 kJ?mol-1��
b��1.6gCH4��ȫȼ������ˮ����ʱ����80.2kJ��1gˮ����ת����Һ̬ˮ����2.444kJ��
��1������������������ϡ��Һ������Ӧ��д���������к��ȵ��Ȼ�ѧ����ʽ��
��2��д����������ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����֪2SO2(g)+O2(g)  2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺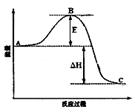
��ͼ��C��ʾ E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ��
�ڸ÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
��Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H���D24.8 kJ?mol-1
�� 3Fe2O3(s)+ CO(g)=2Fe3O4(s)+ CO2(g) ��H���D47.2 kJ?mol-1
��Fe3O4(s)+CO(g)=3FeO(s)+CO2(g) ��H�� +640.5 kJ?mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
_________________________________________________��
(10�֣�
(1) NaOH(aq) +1/2H2SO4 (aq) = 1/2Na2SO4(aq) +H2O(l) ��H=-57.3 kJ?mol-1��2�֣�
(2) CH4(g)+2O2(g)=CO2(g)+2 H2O(l) ��H���D889.98 kJ?mol-1��2�֣�
(3) �� ���������� �ޣ���1�֣���2�֣�
�ڽ��� ��Ϊ�����ı��˷�Ӧ������ʹ���E���ͣ���1�֣���2�֣�
(4) FeO(s)+ CO(g)="=" Fe(s)+CO2(g) ��H��-218.0 kJ?mol-1 ��2�֣�
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012�찲��ʡ��ɽ�и�����У������ѧ�Ծ� ���ͣ������
(14��)X��Y��Z��W��Ԫ�����ڱ���ǰ�����ڵij���Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ԭ�������������Ǵ��������� |
| Z | ���ʼ��仯�������ɫ��ӦΪ��ɫ |
| W | WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
 ��X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣
��X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣 O2(g) ="=" H2O(l)�� ��H= -285.8kJ��mol-1
O2(g) ="=" H2O(l)�� ��H= -285.8kJ��mol-1�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������и߶���һ���¿���ѧ�Ծ��������棩 ���ͣ������
��10�֣���֪��a��H+(aq) + OH-(aq) = H2O(l) ��H=-57.3 kJ•mol-1��
b��1.6gCH4��ȫȼ������ˮ����ʱ����80.2kJ��1gˮ����ת����Һ̬ˮ����2.444kJ��
��1������������������ϡ��Һ������Ӧ��д���������к��ȵ��Ȼ�ѧ����ʽ��
��2��д����������ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����֪2SO2(g)+O2(g)  2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
2SO3(g)��Ӧ���̵������仯��ͼ��ʾ����ش��������⣺
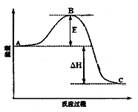
�� ͼ��C��ʾ E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ������Ӱ�죿 ��
�� �÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B�������ǽ��ͣ� �������� ��
��4����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳ�ã�����ͨ����ӵķ����ⶨ���ָ�������3���Ȼ�ѧ��Ӧ����ʽ��
�� Fe2O3(s)+3CO(g)=2Fe(s)+3CO2(g) ��H�� �D24.8 kJ•mol-1
�� 3Fe2O3(s)+ CO(g)=2Fe3O4(s)+ CO2(g) ��H�� �D47.2 kJ•mol-1
�� Fe3O4(s)+CO(g)=3FeO(s)+CO2(g) ��H�� +640.5 kJ•mol-1
д��CO���廹ԭFeO����õ�Fe�����CO2������Ȼ�ѧ��Ӧ����ʽ��
_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ��ɽ�и�����У������ѧ�Ծ� ���ͣ������
(14��)X��Y��Z��W��Ԫ�����ڱ���ǰ�����ڵij���Ԫ�أ��������Ϣ���±���
|
Ԫ�� |
�����Ϣ |
|
X |
X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
|
Y |
ԭ�������������Ǵ��������� |
|
Z |
���ʼ��仯�������ɫ��ӦΪ��ɫ |
|
W |
WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
�� Xλ��Ԫ�����ڱ��� �塣X��һ�ֵ����۵�ܸߣ�Ӳ�Ⱥܴ������ֵ��ʵľ������� ���塣
�� X��Y�е縺�Խ�ǿ����(��Ԫ�ط��ţ� ��XY2�ĵ���ʽ�� �������д��� ���Ҽ���
��Z2Y2�к��еĻ�ѧ�������� �����������ӵĸ�����Ϊ ��
��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ�� ��
�ɷϾ�ӡˢ��·������W�ĵ���A����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·���ϵ�A����֪��
A(s)+H2SO4 (aq) == ASO4(aq) + H2(g)�� ��H=+64.4kJ��mol-1
2H2O2(l) == 2H2O(l) + O2(g)�� ��H= -196.4kJ��mol-1
H2(g)+ O2(g) == H2O(l)�� ��H= -285.8kJ��mol-1
O2(g) == H2O(l)�� ��H= -285.8kJ��mol-1
��д��A��H2SO4��H2O2��Ӧ����ASO4(aq)��H2O(l)���Ȼ�ѧ����ʽ(A�û�ѧʽ��ʾ)��
�� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�����ʡ������У��������������ۣ���ѧ���� ���ͣ�ѡ����
�����й��Ȼ�ѧ����ʽ��������ȷ����
A����֪���������ȶ���С���춡�飬��CH3CH2CH2CH3(g)��(CH3)2CHCH3(g)����H>0
B����֪��
OH-(ag)+H+(aq) H2O(l)����H=-57.4 kJ/mol
H2O(l)����H=-57.4 kJ/mol
��20.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�28.7kJ��������
C����֪��2H2O(l) 2H2(g)+O2(g)����H= +483.6
kJ�� mol��1����������ȼ����Ϊ241.8kJ/mol
2H2(g)+O2(g)����H= +483.6
kJ�� mol��1����������ȼ����Ϊ241.8kJ/mol
D����֪��2C(s)+2O2(g)=2CO2(g)����H1 2C(s)+O2(g)=2CO(g)����H2�����H1>��H2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com