Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��
��1��������ȼ������ʱ������N
2��O
2�ķ�Ӧ��N
2+O
2�T2NO���ǵ�������β���к���NO��ԭ��֮һ��
����T
1��T
2�¶��£�һ������NO�����ֽⷴӦʱN
2�����������ʱ��仯��ͼ����ʾ������ͼ���жϷ�ӦN
2��g��+O
2��g���T2NO��g���ġ�H
0���������������
����T
3�¶��£���2L�ܱ������г���10mol N
2��5mol O
2��50���ﵽƽ�⣬���NO�����ʵ���Ϊ2mol����÷�Ӧ������v��N
2��=
�����¶��£�����ʼʱ�����������г���N
2��O
2��Ϊ1mol����ﵽƽ���N
2��ת����Ϊ
��
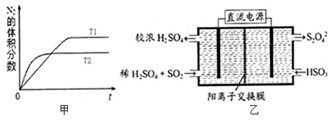
��2��������ͼ����ʾװ�ã��缫��Ϊ���Ե缫��������SO
2���������ų�����Һ������NO
2��
�������ĵ缫��ӦʽΪ
��
���ڼ��������£��������ų�����Һ����NO
2��ʹ��ת��Ϊ�����壬ͬʱ��SO
32-���ɣ��÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ
��
��3��һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol?L
-1�Ĵ�����b mol?L
-1 Ba��OH��
2��Һ�������ϣ���ַ�Ӧ����Һ�д���2c��Ba
2+���Tc��CH
3COO
-������û����Һ�д���ĵ��볣��Ka=
���ú�a��b�Ĵ���ʽ��ʾ����
��4������������PM2.5ϸ���Ӱ�����NH
4��
2SO
4��NH
4NO
3���л������P�ﳾ�ȣ���дһ����SO
42-��Ϊ�ȵ�����ķ���
��

 Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��
Ϊ��Ч�������������ػ�����ȡ��ʩ���ƴ����������о�����Ч���ƿ����еĵ������̼��������������ﺬ���Ե���Ϊ��Ҫ��

 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�