��1��Ϊʵ�ַϾ�ӡˢ��·���ۺ����ã�����H
2O
2��ϡH
2SO
4�Ļ��Һ�ܽ��������ͭ��ĩ�����Ʊ�����ͭ����֪��
��Cu��s��+2H
+��aq��+
O
2��g��=Cu
2+��aq��+H
2O��l����H=-221.45KJ?mol
-1��2H
2O
2��l��=2H
2O��l��+O
2��g����H=-196.46KJ?mol
-1Cu��H
2O
2��ϡH
2SO
4�Ļ��Һ��Ӧ����Cu
2+��H
2O���Ȼ�ѧ����ʽΪ
Cu��s��+H2O2 ��l��+2H+��aq��=Cu2+��aq��+2H2O��l����H=-319.68KJ/mol
Cu��s��+H2O2 ��l��+2H+��aq��=Cu2+��aq��+2H2O��l����H=-319.68KJ/mol
�����ӷ���ʽ��ʽ����
��2�����ʣ�t-BuNO��
2���������ܼ��з������·�Ӧ����t-BuNO��
2��aq��?2��t-BuNO����aq��
�ٵ���t-BuNO��
2����ʼŨ��Ϊ0.50mol/Lʱ��ʵ����20��ʱ��ƽ��ת���ʣ�������65%������20��ʱ������Ӧ��ƽ�ⳣ��K=
2.4
2.4
������С�����һλ��Ч���֣���ͬ����
��һ���¶��º��������У����ţ�t-BuNO��
2����ʼŨ��������ƽ��ת����
��С
��С
������������䡱��С��������֪20��ʱ�÷�Ӧ��CCl
4�ܼ��е�ƽ�ⳣ��Ϊ1.9��������Ӧ�ܼ�������ij�CCl
4�������֣�t-BuNO��
2��ʼŨ����ͬ��������CCl
4�ܼ��е�ƽ��ת����
��
��
������ڡ�����С�ڡ����ڡ��������������ܼ��е�ƽ��ת���ʣ�
��ʵ���ø÷�Ӧ�ġ�H=50.5KJ/mol���÷�Ӧ�ġ�S
��
��
0�����������������=������
�ϸ�
�ϸ�
����ϸߡ��ϵ͡����¶��������ڸ÷�Ӧ�Է����У�




 ���仯�����ڹ���������ռ����Ҫ�ĵ�λ��
���仯�����ڹ���������ռ����Ҫ�ĵ�λ�� ��2012?�Ͳ�һģ�����仯�����ڹ���������ռ����Ҫ�ĵ�λ��
��2012?�Ͳ�һģ�����仯�����ڹ���������ռ����Ҫ�ĵ�λ��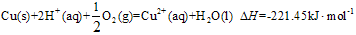

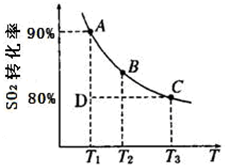
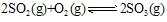 ��һ�������£�SO2��ƽ��ת���ʺ��¶ȵĹ�ϵ��ͼ��ʾ���÷�Ӧ�Ħ�H _______0�����������������Ӧ���е�״̬Dʱ��v��________ v�棨���������������=������?
��һ�������£�SO2��ƽ��ת���ʺ��¶ȵĹ�ϵ��ͼ��ʾ���÷�Ӧ�Ħ�H _______0�����������������Ӧ���е�״̬Dʱ��v��________ v�棨���������������=������? 
 2SO3(g)��ƽ�ⳣ��K=_________��
2SO3(g)��ƽ�ⳣ��K=_________��