
| |||||||||||||||

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʦ���и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
����6�֣������������ʣ� ��NaCl���塡��Һ̬SO2���۴����ᡡ�����ᱵ����ͭ �ƾ���C2H5OH�� ���ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ�������������������������������������������������� ��
��2������������ʵ������������������������������������� ��
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ������������������������������ ��
����4�֣�
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð�
������ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��
A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ��
��ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ
������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
 H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH  H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ԫ��������������ʼ�ת���ij���Ԫ�ء�
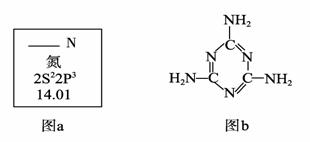
��1��ͼa��Ԫ�����ڱ���NԪ�ص��й���Ϣ��ͼa�к�����ȱʧ�ľ����������������� ��
15N��NԪ�ص�һ����Ҫ���أ���һ��ԭ�Ӻ��к��е�������Ϊ�������� ��
��2�������谷�Ľṹ��ʽ����ͼb����������˽⣬���й��������谷�ı����У�
��ȷ������������ (����ĸ���)��
A�������谷���۵���ܸܺߡ� B�������谷�ĺ������ߴ�67%����
������ C�������谷����������� D�������谷����������ԭ����ͬһƽ����
��3���������ѧ���Ǻϳ����ɵ�Ԫ���γɵ�N5n+������ʽ![]() Ϊ
Ϊ
��nֵΪ������ ��
��H��N���γɻ�����NH5 ����֪��������ˮ��Ӧ��H2���ɣ���NH5�к��еĻ�ѧ��Ϊ���������������� ��
��4����֪MΪ��Ԫ����һ�ֽ���Ԫ����ɵ����ӻ�������н���Ԫ�ص���������Ϊ
35��4����M��������ȫ����������Ҫ�����á�������GΪ����ɫ���塣HΪһ�ֳ�����Һ�壬A��B��C��XΪ���ʣ�����A��C��XΪ���壬A��X��Ϊ�����гɷ֡�I��JΪ�����Ĺ�ҵԭ�ϡ�

��д��D��X��Ӧ�Ļ�ѧ����ʽ����������������������������������
��д��G��H��Ӧ�����ӷ���ʽ������������������������������������ ��
��ʵ������IӦ��α��棿�� ������������������������������ ��
�ܳ�����M��ײ��ʱ�ɷֽ⣬13gM��ȫ�ֽ�ΪA��Bʱ���ų�akJ��������д��M�ֽ���Ȼ�ѧ����ʽ�������������������������������������������� ��
��M��һ��������ˮ���Σ���ˮ��Һ�������ԣ������ӷ���ʽ����ԭ������������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ�и������е���ĸ��ʾ�йص�һ�ַ�Ӧ���������(ijЩ������ȥ)���г�����B��D��G��I��JΪ���壬����B��ʹʪ��ĺ�ɫʯ����ֽ������A��N������������ֻ��GΪ���ʣ�����Ϊ�����NΪ������ˮ�����ᡣ

�ش��������⣺
(1)A������Ϊ����������������������������F�Ļ�ѧʽ������������������H�Ļ�ѧʽ������������������L�Ļ�ѧʽ������������������ ������ ��������
(2)д��ʵ������ȡI�����ӷ���ʽ���� �������������������������������������������������������������� ������������������������������������������������
(3)д��G��һ��ͬ��������Ļ�ѧʽ������������������������G�ڻ�ѧ�����ϵĹ�ͬ����������������������������
(4)��֪��ҵ������0.1molB�ų�4.62kJ������д���÷�Ӧ���Ȼ�ѧ����ʽ��
������ ������������������������������������������������������������������������ ��������������������������������������������������������������
(5)�ڷ�ӦC��E��G��F�У�ÿ����1molGת����������������mol���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�»���2009��10��15�ձ�����ȫ��ũ��Ӧ���ڡ���ɫ��̬��������ʡ���̼���ܡ�ѭ����չ�������������£����ø���ط�չ���й���ɫ��ׯ�������롰��̫���ʵ�̼ũׯ�����衣�ɼ�����̼ѭ�����Ѿ������˹�������ӣ��Իش��������⣺
�� ��1��ú��������Һ���������ȼ�ϵ������ʡ�
������ ��֪25�棬101kPaʱ��C��s��+![]()
������ 
������ ����25�棬101kPaʱ��C��s��+H2O��g��=CO��g��+H2��g����H=������ ��
�� ��2����¯������CO�������Ҫ��;֮һ���������ӦΪ��
������ FeO��s��+CO��g�� ![]() ���� Fe��s��+CO2��g����H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
���� Fe��s��+CO2��g����H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
���������¶����ߣ���ѧƽ���ƶ���ﵽ�µ�ƽ�⣬��ʱƽ�ⳣ��K��ֵ���� �����������С�����䡱��
��1100��ʱ��ø�¯�У�c(CO2)=0.025mol��L-1��c(CO)=0.1mol��L-1��������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�������� ����ǡ��������ж�������
���������������������������������������������������������� ��
�� ��3��Ŀǰ��ҵ�Ͽ���CO2������ȼ�ϼ״����йط�ӦΪ��
��������CO2��g��+3H2��g�� ![]() ���� CH3OH��g��+H2O��g����H=��49.0kJ��mol-1���������Ϊ1L���ܱ������У�����1molCO2��3molH2����Ӧ�����в��CO2��CH3OH��g����Ũ����ʱ��ı仯��ͼ��ʾ��
���� CH3OH��g��+H2O��g����H=��49.0kJ��mol-1���������Ϊ1L���ܱ������У�����1molCO2��3molH2����Ӧ�����в��CO2��CH3OH��g����Ũ����ʱ��ı仯��ͼ��ʾ��
|
������ �ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)=������ ��
�����д�ʩ��ʹ![]() ������������� ������ţ���
������������� ������ţ���
A�������¶ȡ��������������������������������� B���ٳ���H2
C���ٳ���CO2�������������������������������� D����H2O��g������ϵ�з���
E������He��g����ʹ��ϵѹǿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��Ŀ����ѧ�����ͨѶ����ͨ���ճ����������Ź㷺��Ӧ�á�
ǰ���õ�����Ni���ӣ�Cd����أ������ܷ�Ӧ���Ա�ʾΪ��
Cd��2NiO(OH)��2H2O�������� ![]() 2Ni(OH)2��Cd(OH)2
2Ni(OH)2��Cd(OH)2
��1����֪Ni(OH)2��Cd(OH)2��������ˮ���������ᣬ�ŵ����ʹ�õ�صĹ��̣�����Ǹ���ز�������Ĺ��̡�����˵������ȷ��������
�� ���Ϸ�Ӧ�������û���Ӧ�� �� ���Ϸ�Ӧ�Ǹ��ֽⷴӦ
�� ���ʱ��ѧ��ת��Ϊ���ܡ� �� �ŵ�ʱ��ѧ��ת��Ϊ����
A���٢ۡ� ������������ B���ڢܡ� ���������� C���٢ܡ� ���������� D���ڢ�
��2�����������ӵ���ѳ�Ϊ��Ҫ�Ļ�����Ⱦ������ϱ���һ�ڷ�������ؿ���ʹһƽ��������ĸ���ʧȥʹ�ü�ֵ��������������������Ⱦ��Ϊ���ء�������Ϊ���������� ����������������������������������������������������������������
��3����һ�ֳ��õĵ����﮵�أ�����һ�ּ����Ԫ�أ������ԭ������Ϊ7���������ı���������λ��������������ת���ĵ������ر����㷺Ӧ��������������һ��ʹ�õ�ʱ��ɳ���ʮ�ꡣ���ĸ����ý�����Ƴɣ�����ܷ�Ӧ�ɱ�ʾΪ��Li��MnO2��LiMnO2�Իش�﮵�ر������ر���ԭ��������������������������������������������������
﮵���еĵ������Һ���÷�ˮ�ܼ����ƣ�Ϊʲô���ֵ�ز���ʹ�õ���ʵ�ˮ��Һ�����û�ѧ����ʽ��ʾ��ԭ��������������������������������������������������������������
��4����ͼ�Ƿ������ֵ���е��ؽ�������ˮ����������һ��;����
������D���ؽ���Ũ������ �����ڡ����ڡ�С�ڣ�������A���ؽ���Ũ�ȣ�����ͨ��ʳ������������ ����������ġ�������;���⣬����Ⱦˮ���е��ؽ���������ֱ��ͨ������������ ��;����_________�������塣
��5����ͼ�������������ϵ�˵������ij�ͺŽ��ڵ��
ij�ͺŽ��ڵ�� | ij�ͺŹ������ |
| GNY 0.6��KR��AA�� |
�������ڵ�صĵ綯���������� ������������������ɷų������� ����ʱ�ĵ��������õ��ƽ����������Ϊ0.03����������ʹ�������� Сʱ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com