
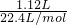 =0.05mol������̼Ԫ���غ��֪n��CO32-��=n��CO2��=0.05mol��
=0.05mol������̼Ԫ���غ��֪n��CO32-��=n��CO2��=0.05mol��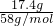 =0.3mol������þԪ���غ��֪n��Mg2+��=n[Mg��OH��2]=0.3mol����þ���ӵ����ʵ���Ϊ0.3mol��
=0.3mol������þԪ���غ��֪n��Mg2+��=n[Mg��OH��2]=0.3mol����þ���ӵ����ʵ���Ϊ0.3mol�� ��0.45L��2mol/L-0.3mol��2��=0.1mol������0.05mol��y=0.1mol����y=2��
��0.45L��2mol/L-0.3mol��2��=0.1mol������0.05mol��y=0.1mol����y=2��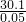 =602����24��6+27��2+17��16+60+18n=602�����n=4��
=602����24��6+27��2+17��16+60+18n=602�����n=4�� ���������̼�����ʵ���������̼Ԫ���غ����̼��������ʵ�����
���������̼�����ʵ���������̼Ԫ���غ����̼��������ʵ����� ����������þ�����ʵ���������þԪ���غ����þ���ӵ����ʵ�����
����������þ�����ʵ���������þԪ���غ����þ���ӵ����ʵ�����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ��УЭ���������ѧ���ڳ����Ի�ѧ�Ծ��������棩 ���ͣ�������
С�մ�θ��ƽ����ϲ���dz��õ��к�θ���ҩ�
��1��С�մ�ÿƬ��0��50gNaHCO3,2ƬС�մ�Ƭ��θ����ȫ�кͣ����к͵�H+�� mol��
��2��θ��ƽÿƬ��0��245gAl(OH)3���к�θ��ʱ��6ƬС�մ��൱��θ��ƽ Ƭ��
��3����ϲ�Ļ�ѧ�ɷ�������þ�ļ�ʽ�Σ�ȡ�ü�ʽ��3��01g,����2��0mol/L����ʹ���ܽ⣬����������42��5mLʱ��ʼ����CO2������������45��0mLʱ������ȫ��Ӧ������ü�ʽ����Ʒ����������̼��������ʵ���֮�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ���������δ�ѧ�Ծ��������棩 ���ͣ�������
С�մ�θ��ƽ����ϲ���dz��õ��к�θ���ҩ�
��1��С�մ�ÿƬ��0��50gNaHCO3,2ƬС�մ�Ƭ��θ����ȫ�кͣ����к͵�H+�� mol
��2��θ��ƽÿƬ��0��245gAl��OH��3���к�θ��ʱ��6ƬС�մ��൱��θ��ƽ Ƭ
��3����ϲ�Ļ�ѧ�ɷ�������þ�ļ�ʽ�Σ�
ȡ�ü�ʽ��3��01g,����2��0mol/L����ʹ���ܽ⣬����������42��5mLʱ��ʼ����CO2������������45��0mLʱ������ȫ��Ӧ������ü�ʽ����Ʒ����������̼��������ʵ���֮��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com