
���÷Ͼɶ�п��Ƥ���Ʊ�����Fe3O4�������Ӽ�������ZnO���Ʊ�����ͼ���£�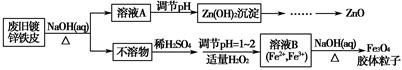
��֪��Zn���仯�����������Al���仯������������ơ���ش��������⣺
��1����NaOH��Һ�����Ͼɶ�п��Ƥ��������________��
| A��ȥ������ | B���ܽ��п�� | C��ȥ������ | D���ۻ� |
 �����߿����ϵ�д�
�����߿����ϵ�д� �㾦�½̲�ȫ�ܽ��ϵ�д�
�㾦�½̲�ȫ�ܽ��ϵ�д� Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ۻ�(As4S4)�ʹƻ�(As2S3)����ȡ�����Ҫ����ԭ�ϣ���������Ȼ����
�����������������������գ�
(1)As2S3��SnCl2�������з�Ӧת��ΪAs4S4��SnCl4���ų�H2S���塣��As2S3��SnCl2������ȫ��Ӧ��As2S3��SnCl2�����ʵ���֮��Ϊ________��
(2)������Ӧ�е���������________����Ӧ�������������________���ա�
(3)As2S3��HNO3�����·�Ӧ��As2S3��10H����10NO3��=2H3AsO4��3S����10NO2����2H2O��������2 mol H3AsO4����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ________�������÷�Ӧ��Ƴ�һԭ��أ���NO2Ӧ����________(�������������)�����ݳ���
(4)����Ӧ����NO2��11.2 L O2(��״��)��Ϻ���ˮ����ȫ��ת����Ũ���ᣬȻ���������C��Ӧ����������CO2����________(ѡ����)��
a����0.5 mol b������0.5 mol
c������0.5 mol d����ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��100 mL FeI2��Һ����ͨ��Cl2������������I2��Fe3����IO3-������Fe3����I2�����ʵ�����n(Cl2)�ı仯��ͼ��ʾ����ش��������⣺
��1����ͼ��֪��I����Fe2����I2�������ӵĻ�ԭ����ǿ������˳��Ϊ________��________��________��
��2����n(Cl2)��0.12 molʱ����Һ�е�������ҪΪ________________________________��
�ӿ�ʼͨ��Cl2��n(Cl2)��0.12 molʱ���ܷ�Ӧ�Ļ�ѧ����ʽΪ______________________��
��3������Һ��n(Cl��)��n(IO3-)��8��1ʱ��ͨ���Cl2�ڱ�״���µ����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͭ��������������Ԫ�أ�Ҳ����������ʹ�õĽ���֮һ��ͭ��������ʹ�öԹ��������������涼��������Զ��Ӱ�졣
��1��д��ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ��________________________________________________________________________________________________________________________________________________��
��2��Ϊ�˱��������ͽ�Լ��Դ��ͨ������H2O2��ϡ����Ļ����Һ�ܳ��Ͼ�ӡˢ��·���е�ͭ������ʵ��ͭ�Ļ������á�д���ܳ�ͭ�����ӷ���ʽ��________________________________________________________________________________________________________________________________________________��
��3����ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O��Cu2S 6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol��
6Cu��SO2�����÷�Ӧ����������______________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ���Ϊ__________mol��
��4��ͭ�ڳ�ʪ�Ŀ������ܷ���������ʴ�����⣬ͭ�����Ҫ�ɷ�ΪCu2��OH��2CO3����ʽ̼��ͭ������д�����������и����ĵ缫��Ӧʽ��________________________________________________________________________________________________________________________________________________��
��5���о���ѧϰС���á���ӵ��������ⶨij����CuSO4��5H2O����������I����Ӧ�����������ʣ��ĺ�����ȡa g�������100 mL��Һ��ÿ��ȡ25.00 mL���μ�KI��Һ���а�ɫ�⻯��������ɡ�д���÷�Ӧ�����ӷ���ʽ��___________________________�������μ�KI��Һ���������ٲ�������Һ�е�I2����������Ʊ���Һ�ζ���������Ӧ�Ļ�ѧ����ʽΪI2��2Na2S2O3=2NaI��Na2S4O6��ƽ������c mol/L��Na2S2O3��ҺV mL����������CuSO4��5H2O����������Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������������������ˮ��ҵ��ˮ����������������������Ͽ졣ʵ���ҿ��ö�������Ϊ��Ҫԭ���Ʊ�������ء��䲿���������£�
(1)�ڢٲ��в��������������ô�������ԭ����(�û�ѧ����ʽ��ʾ)________________________________________��
(2)KOH��KClO3��MnO2���۷�Ӧ����ī��ɫK2MnO4�Ļ�ѧ����ʽΪ________________________________________��
(3)�ڢܲ�ͨ��CO2����ʹMnO42��������Ӧ������MnO4����MnO2����K2MnO4��ȫ��Ӧʱ��ת��ΪKMnO4�İٷ���ԼΪ________(��ȷ��0.1%)��
(4)�ڢݲ����ȹ��˵�Ŀ����______________________________________��
(5)�ڢ�����Ũ����Һ����ϸС��������ʱ��ֹͣ���ȣ���ȴ�ᾧ��________��ϴ�ӡ������������У��¶Ȳ��˹��ߣ���Ϊ____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�й�����ֵ�������ʾ��2013��ȫ��ƽ����������Ϊ52����֮��γ���������Ҫ�ɷ�Ϊ�������������ŷŵķ���������β�����ﳾ�ȡ�
��1����CH4������������β���е����������Ⱦ��
��֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(l) ��H����955 kJ/mol
2NO2(g)��N2O4(g) ��H����56.9 kJ/mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ ��
��2����֪��CO(g)��H2O(g) CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g)
CO2(g)��H2(g) ��H����41kJ/mol��ij�¶��£����ݻ�Ϊ2L���ܱ������г���2.0molCO(g)��2.0molH2O(g)����tminʱ�ﵽƽ�⣬��÷ų���32.8kJ��������tmin����H2��ʾ��ƽ����Ӧ����Ϊ ���ɴ˿�֪�ڸ��¶��·�ӦCO2(g)��H2(g) CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ��
CO(g)��H2O(g)�Ļ�ѧƽ�ⳣ��Ϊ ����ͬ�����£���ͬһ�ܱ������г���1.0molCO2��1.0molH2��Ӧ�ﵽƽ������յ�����Ϊ kJ��
��3����ʽ��������������������Ҫ����������
����Al2(SO4)3��Һ��Ͷ���ĩ״ʯ��ʯ�����ɼ�ʽ������[Al2(SO4)3��Al2O3]��Һ��
�ڼ�ʽ����������SO2��Al2(SO4)3��Al2O3+3SO2��Al2(SO4)3��Al2(SO3)3����д��Al2(SO4)3��Al2O3������ռ���Һ��Ӧ�Ļ�ѧ����ʽ ��
�۽�Al2(SO4)3��Al2 (SO3)3������Al2(SO4)3����ѡ��������Ϊ ������ţ�
| A��Ũ���� | B��KMnO4��Һ | C��5%��H2O2��Һ | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
H2O2�ڹ�ҵ��ũҵ��ҽҩ�϶��й㷺����;��
��1��H2O2�Ƕ�Ԫ���ᣬд����һ���ĵ��뷽��ʽ ���ڶ����ĵ���ƽ�ⳣ������ʽKa2�� ��
��2���������ʶ�������H2O2�ֽ�Ĵ�����һ�ֹ۵���Ϊ���ڷ�Ӧ�����д����ȱ�H2O2��������ԭ�������ֱ�H2O2��ԭ�������������������ʶ�����H2O2�ֽ�Ĵ������ڷ�Ӧ�������ȱ���������ԭ���� ��
��I�� ��Fe3�� ��Cu2�� ��Fe2��
��3���ü�������ȼ�ϵ�غϳ�H2O2������Ч�ʸߣ�����Ⱦ���ص㡣����ܷ�ӦΪ��
H2 + O2 + OH�� �� H2O + HO2����д��������Ӧʽ�� ��
��4��H2O2��һ�ֻ����Ѻõ�ǿ����������Ʒ�ˮ����Ҫ��Cu2����Ni2������������Fe3����Fe2����Cr3�� �ȣ��Ʊ���������һ���������£�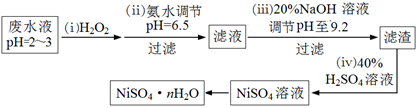
�ٵڣ�������������H2O2��Ӧ�����ӷ���ʽ ��
�ڵڣ��������������е���Ҫ�ɷ���ҽ���ϵ���;�� ��
��Ϊ�ⶨNiSO4��n H2O����ɣ���������ʵ�飺��ȡ2.627g��Ʒ�����Ƴ�250.00 mL��Һ��ȷ��ȡ���Ƶ���Һ25.00 mL����0.04000 mol��L��1��EDTA��Na2H2Y������Һ�ζ�Ni2+�����ӷ���ʽΪNi2��+ H2Y2����NiY2��+ 2H����������EDTA����Һ25.00 mL��������������Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�NO2��SO2��CO�ȴ�����Ⱦ����Ĵ���������Ҫ���塣
��1�����÷�Ӧ��6NO2��8NH3 7N2��12H2O�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״���µ������ L��
7N2��12H2O�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״���µ������ L��
��2������֪��2SO2(g)+O2(g) 2SO3(g) ��H��?196.6 kJ��mol�C1
2SO3(g) ��H��?196.6 kJ��mol�C1
2NO(g)+O2(g) 2NO2(g) ��H��?113.0 kJ��mol�C1
2NO2(g) ��H��?113.0 kJ��mol�C1
��д��NO2��SO2��Ӧ����SO3(g)��NO���Ȼ�ѧ����ʽ ��
��һ�������£���NO2��SO2�������1:2�����ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
a����ϵѹǿ���ֲ��� b�����������ɫ���ֲ���
c��SO3��NO������ȱ��ֲ��� d��ÿ����1 mol SO2��ͬʱ����1 molNO2
�۲��������Ӧƽ��ʱNO2��SO2�����Ϊ1:6����÷�Ӧ��ƽ�ⳣ��K�� ��
��3������β���е�һ����̼��ͨ�����·�Ӧ������Ũ�ȣ�CO(g)+1/2O2(g) CO2(g)����֪ij�¶��£������������ܱ������н��и÷�Ӧ�������и����ʵ���ʼŨ�ȼ������淴Ӧ���ʹ�ϵ���±���ʾ�����á���������������д���еĿո�
CO2(g)����֪ij�¶��£������������ܱ������н��и÷�Ӧ�������и����ʵ���ʼŨ�ȼ������淴Ӧ���ʹ�ϵ���±���ʾ�����á���������������д���еĿո�
| ������� | c(CO)��mol��L�C1 | c(O2)��mol��L�C1 | c(CO2)��mol��L�C1 | ��(��)�ͦ�(��) ��С�Ƚ� |
| �� | 2.0��10�C4 | 4.0��10�C4 | 4.0��10�C4 | ��(��)����(��) |
| �� | 1.0��10�C3 | 4.0��10�C4 | 5.0��10�C4 | ��(��) ��(��) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��
Ư���dz��õ�����������ҵ�Ͻ�����ͨ��ʯ����[Ca(OH)2]��ȡƯ�ۣ���ѧ��Ӧ����ʽΪ ��Ư�۵���Ч�ɷ��ǣ��ѧʽ�� ���÷�Ӧ���������뻹ԭ�����ʵ���֮���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com