
 ČēĶ¼ĖłŹ¾£¬PĪŖŅ»æÉ×ŌÓÉ»¬¶ÆµÄ»īČū£¬¹Ų±ÕK£¬·Ö±šĻņČŻĘ÷A”¢BÖŠ¾łø÷³äČė2mol X”¢2mol Y£¬ĘšŹ¼Ź±£¬VA=a L£¬VB=0.8a L£ØĮ¬ĶعܵÄĢå»żŗöĀŌ²»¼Ę£©£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀŹö·“Ó¦£ŗ3X£Øg£©+3Y£Øg£©?2Z£Øg£©+2W£Øg£©£¬“ļµ½Ę½ŗāŹ±£¬VB=0.6a L£®
ČēĶ¼ĖłŹ¾£¬PĪŖŅ»æÉ×ŌÓÉ»¬¶ÆµÄ»īČū£¬¹Ų±ÕK£¬·Ö±šĻņČŻĘ÷A”¢BÖŠ¾łø÷³äČė2mol X”¢2mol Y£¬ĘšŹ¼Ź±£¬VA=a L£¬VB=0.8a L£ØĮ¬ĶعܵÄĢå»żŗöĀŌ²»¼Ę£©£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō·¢ÉśĻĀŹö·“Ó¦£ŗ3X£Øg£©+3Y£Øg£©?2Z£Øg£©+2W£Øg£©£¬“ļµ½Ę½ŗāŹ±£¬VB=0.6a L£®| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| 3 |
| 1.5mol |
| 2mol |


 ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
ĘŚÄ©¼Æ½įŗÅĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
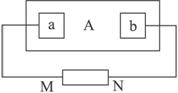
£Ø1£©µēŌ“ÄÄŅ»¼«ŹĒÕż¼«£æ__________________________________________
£Ø2£©bÖÜĪ§æɹŪ²ģµ½Ź²Ć“ĻÖĻó£æĪŖŹ²Ć“£æ__________________________________________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
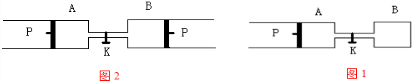
ČēĶ¼ĖłŹ¾£¬PĪŖŅ»æÉ×ŌÓÉ»¬¶ÆµÄ»īČū£¬¹Ų±ÕK£¬·Ö±šĻņČŻĘ÷A”¢BÖŠø÷³äČė2mol X”¢2molY£¬ĘšŹ¼Ź±£¬VA=![]() L£¬VB=0.8
L£¬VB=0.8![]() L(Į¬ĶعܵÄĢå»żŗöĀŌ²»¼Ę)£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō¾ł·¢ÉśĻĀŹö·“Ó¦£ŗ
L(Į¬ĶعܵÄĢå»żŗöĀŌ²»¼Ę)£¬ŌŚĻąĶ¬ĪĀ¶ČŗĶ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ£¬Į½ČŻĘ÷ÖŠø÷×Ō¾ł·¢ÉśĻĀŹö·“Ó¦£ŗ
3X(g)+3Y(g)![]() 2Z(g)+2W(g)£¬“ļµ½Ę½ŗāŹ±£¬VB=0£®6
2Z(g)+2W(g)£¬“ļµ½Ę½ŗāŹ±£¬VB=0£®6![]() L”£
Lӣ

(1)BÖŠXµÄ×Ŗ»ÆĀŹĪŖ ”£
(2)A”¢BÖŠXµÄ×Ŗ»ÆĀŹµÄ¹ŲĻµŹĒA B(Ģī”°>”±”°=”±»ņ”°<”±)£¬ĘäĄķÓÉŹĒ ”£
(3)Ę½ŗāŹ±A”¢BÖŠ»ģŗóĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæµÄ¹ŲĻµŹĒ£ŗMA MB(Ģī”°>”±”°=”±»ņ”°<”±)
(4)“ņæŖK£¬Ņ»¶ĪŹ±¼äŗó·“Ó¦ŌŁ“Ī“ļµ½Ę½ŗā£¬Čē¹ūŅŖ¼ĘĖć³ö“ĖŹ±BČŻĘ÷ÖŠ»ģŗĻĘųĢåµÄĆܶȔ£ŌņÖĮÉŁ»¹ŠčŅŖÖŖµĄŅŌĻĀŹż¾Ż×éŗĻÖŠµÄ ×é(ĢīŃ”Ļī×ÖÄø)”£(ÉčMX”¢MY”¢MZ”¢MW·Ö±š±ķŹ¾X”¢Y”¢Z”¢WµÄĦ¶ūÖŹĮæ)
A£®MzŗĶMw B£®MXŗĶMY C£®MxŗĶMz D£®MYŗĶMW
Š“³öĘäĆܶȵÄŅ»ÖÖ±ķ“ļŹ½£ŗ g”¤L![]() ”£
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com