
�����������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����2�֣�CH3������CH3��CH3��������Ҫ���л���Ӧ�м��壬�й����ǵ�˵����ȷ����
A�����Ǿ��ɼ���ȥ��һ����ԭ������
B�����ǵĿռ乹����ͬ��̼ԭ�Ӿ����Բ�ȡsp2�ӻ�
C��CH3����NH3��H3O+��Ϊ�ȵ����壬���ι��;�Ϊ������
D��CH3���е�̼ԭ�Ӳ�ȡsp3�ӻ�������ԭ�Ӿ�����
��2����3�֣��ڼ��Է����У����������ͬ��������ļ�ľ����ż������ͨ����d��ʾ�����Է��ӵļ���ǿ��ͬż������������������ĵĵ�����q���йأ�һ����ż���أ��̣������������ӵ�ż���ض���Ϊż������ż����һ�˵�ɵ����ij˻������̣�d��q���Իش��������⣺
��HCl��CS2��H2S��SO2���ַ����Ц̣�0���� ��
��ʵ���ã���PF3��1.03����BCl3��0���ɴ˿�֪��PF3������ ���ͣ�BCl3������ ���͡�
��3����2�֣�![]() �����ʵĴ����о����������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���__________________��
�����ʵĴ����о����������������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ���__________________��
 ��4����3�֣����ù��������֤ʵ���ڽྻ���������ںϳɰ�
��4����3�֣����ù��������֤ʵ���ڽྻ���������ںϳɰ�
��Ӧ�Ĵ������ı����ϴ��ڵ�ԭ�ӣ���ͼΪ��ԭ����
���ľ����ϵĵ��㸽�žֲ�ʾ��ͼ��ͼ��С��ɫ�����
��ԭ�ӣ���ɫ�������ԭ�ӣ������ڵ��㾧����N/Feԭ
����֮��Ϊ________________��
��5����2�֣���������Ľṹ���õȾ�Բ����ܶѻ����������ڵȾ�Բ������ܶѻ��ĸ�����ʽ�У��������ܶѻ����������ܶѻ���Ϊ��Ҫ����ָ����ͼ���ĸ�Ϊ�������ܶѻ� ���A����B����
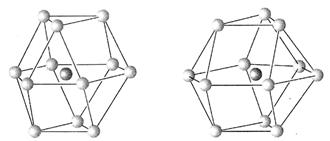
ͼA ͼB

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���ۼ� | C-C | C-N | C-S |
| ����/kJ?mol-1 | 347 | 305 | 259 |
 ��3����ѧ��ͨ��X����̽����KCl��MgO��CaO��TiN�ľ���ṹ��NaCl�ľ���ṹ���ƣ���ͼһ��ʾ��������3�����Ӿ���ľ������������±���
��3����ѧ��ͨ��X����̽����KCl��MgO��CaO��TiN�ľ���ṹ��NaCl�ľ���ṹ���ƣ���ͼһ��ʾ��������3�����Ӿ���ľ������������±���| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ?mol-1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?μ�϶�ģ�������������ʵ���Ҫ���������ʽṹ����ش��������⣮
��2013?μ�϶�ģ�������������ʵ���Ҫ���������ʽṹ����ش��������⣮| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���ۼ� | C-C | C-N | C-S |
| ����/kJ?mol-1 | 347 | 305 | 259 |
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ?mol-1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?�����ж�ģ�������������ʵ���Ҫ���������ʽṹ����ش��������⣮
��2011?�����ж�ģ�������������ʵ���Ҫ���������ʽṹ����ش��������⣮| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ?mol-1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
| ������/kJ��mol��1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
��A�Ļ��ϼ� B�Ļ��ϼۣ����������������������
��2��ʵ��֤����KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ�����ͼ��ʾ��������3�����Ӿ���ľ������������±���
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |
��� 4�����Ӿ��壨������NaCl���۵�Ӹߵ��͵�˳���ǣ� ������MgO������һ��Mg2����Χ�������ڽ��ҵȾ����Mg2���� ����
��3�����������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ��� ��
��4��ij�����ķ��ӽṹ����ͼ��ʾ����Nԭ�ӵ��ӻ���ʽΪ ����̬Niԭ�ӵĵ����Ų�ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ��10�·��¿���ѧ�Ծ� ���ͣ������
��12�֣������������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
|
������/kJ��mol��1 |
I1 |
I2 |
I3 |
I4 |
|
A |
578 |
1817 |
2745 |
11578 |
|
B |
738 |
1451 |
7733 |
10540 |
����A�Ļ��ϼ����������� B�Ļ��ϼۣ����������������������
��2��ʵ��֤����KCl��MgO��CaO��TiN��4�־���Ľṹ��NaCl����ṹ���ƣ�����ͼ��ʾ����

����3�����Ӿ���ľ������������±���
|
���Ӿ��� |
NaCl |
KCl |
CaO |
|
������/kJ��mol��1 |
786 |
715 |
3401 |
��� 4�����Ӿ��壨������NaCl���۵�Ӹߵ��͵�˳���ǣ����������� ��ԭ���ǣ� �� ��
��3�����������Ӻ�δ�ɶԵ���Խ�࣬�����Խ�ż�¼����Խ�á�������������V2O5��CrO2�У��ʺ���¼�����ŷ�ԭ�ϵ������������� ��
��4��ij�����ķ��ӽṹ����ͼ��ʾ��

��Nԭ�ӵ��ӻ���ʽΪ ������ ����̬Niԭ�ӵĵ����Ų�ʽ���������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com