
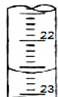
 ��ijѧ����0.20mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����
��ijѧ����0.20mol•L-1�ı�NaOH��Һ�ζ�δ֪Ũ�ȵ����ᣬ�����Ϊ���¼�����| �ζ����� | ���������ml�� | ���ռ������ml�� | |
| �ζ�ǰ���� | �ζ������ | ||
| ��һ�� | 20.00 | 0.40 | 20.40 |
| �ڶ��� | 20.00 | 4.00 | 24.00 |
| ������ | 20.00 | 2.00 | 24.10 |
���� ��1���ζ�����װҺǰҪ������ϴ��
��2������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��3�����ݵζ��ܵĽṹ�;�ȷ��Ϊ0.01mL�����
��4���ȸ������ݵ���Ч�ԣ���ȥ��3�����ݣ�Ȼ�����1��2��ƽ������V��NaOH�������Ÿ��ݸ���c�����⣩=$\frac{c��������V������}{V�����⣩}$�����㣮
��5�����û����ҺΪ���ԣ�һ������n��H+��=n��OH-�������¶���ˮ�����ӻ�����KW=10-13����pH=11����Һ��c��OH-��=$\frac{1{0}^{-13}}{1{0}^{-11}}$mol/L=0.01mol/L��pH=1��������������Ũ��Ϊ0.1mol/L���ݴ���ʽ���㣻
��6���ṹ���Ƶ��������Σ��ܶȻ�ԽС���ܽ��ԽС��AgY�ܵ��ܶȻ���AgXС���ӳ���ƽ���ƶ��ĽǶȷ�����
��� �⣺��1���ζ�����װҺǰҪ������ϴ�����Ԣٴ���
�ʴ�Ϊ���٣�
��2��������У��ڼ��µζ���Һ�����ʱ���ζ��ܼ��������ݣ����V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������֪c������ƫС��
�ʴ�Ϊ��ƫС��
��3���ζ�ʱ�ĵζ����е�Һ�棬�����Ϊ22.60mL��
�ʴ�Ϊ��22.60��
��4�����εζ����ĵ����Ϊ��20.00mL��20.00mL��22.10����ȥ��3�����ݣ�Ȼ�����1��2��ƽ������V��NaOH��=20.00mL��c�����⣩=$\frac{c��������V������}{V�����⣩}$=$\frac{0.20mol•{L}^{-1}��20.00mL}{20.00mL}$=0.2000mol•L-1��
�ʴ�Ϊ��0.2��
��5�����û����ҺΪ���ԣ���n��H+��=n��OH-�������¶���ˮ�����ӻ�����KW=10-13����pH=11����Һ��c��OH-��=$\frac{1{0}^{-13}}{1{0}^{-11}}$mol/L=0.01mol/L��
pH=1��������������Ũ��Ϊ0.1mol/L��
��bL��0.1mol/L=aL��0.01mol/L��
��ã�a��b=10��1
�ʴ�Ϊ��10��1��
��6�����ݻ�ѧʽ���Ƶ����ʵ��ܶȻ�����ԽС������Խ���ܣ��ܽ��ԽС����֪�����ܽ�ȣ�mol/L���Ĵ�С˳��ΪS��AgX����S��AgY����S��AgZ����
AgX��AgY�ܽ�ȴ�����AgX������Һ��c��Ag+���ϴ�����AgY�ı�����Һ�м�������AgX�Ĺ��壬��c��Ag+������AgY�ij����ܽ�ƽ�����ƣ�����c��Y-������С��
�ʴ�Ϊ��s��AgX����s��AgY����s��AgZ������С��
���� ���⿼���к͵ζ�����ҺpH�ļ��㡢���ܵ���ʵ��ܽ�ƽ�⼰����ת���ı��ʣ�����ѧ���ķ��������������Ŀ��飬��Ŀ�Ѷ��еȣ�����ע���ܶȻ����������ú����⣮


 ��У���һ��ͨϵ�д�
��У���һ��ͨϵ�д� �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
ȫ�ܴ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ۢܢݢ� | D�� | ȫ����ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | þԪ����Ԫ�����ڱ���λ�ڵ������ڡ��ڢ�B�� | |
| B�� | �ڢڲ�ϴ����Ϻ���ϴ��Һ�еμ�̼������Һ�ɼ�������Ƿ�ϴ�Ӹɾ� | |
| C�� | �ڹ�ҵ�����Ͽ���NaOH��Һ����ʯ���� | |
| D�� | �����Ҳ���Բ��õ��þ���ˮ��Һ�ķ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
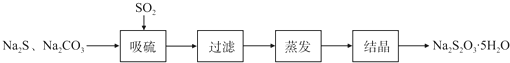
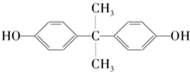 ��������̼�����Ĺ������£�
��������̼�����Ĺ������£�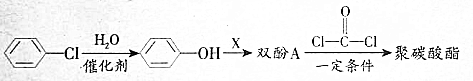
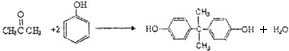 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
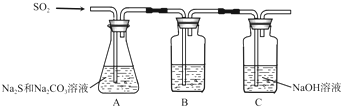
| ��� | ʵ����� | Ԥ������ | ���� |
| �� | ȡ������Ʒ���Թ��У�������������ˮ��������ܽ⣬�μ�����ϡ���ᣬ�ٵμ�����AgNO3��Һ���� | �а�ɫ�������� | ��Ʒ��NaCl |
| �� | ��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬��ȡ������Ʒ���ձ��У�������������ˮ����ֽ����ܽ⣬�������CaCl2��Һ�����裬���ã���pH�Ʋⶨ�ϲ���ҺpH | �а�ɫ�������ɣ��ϲ���ҺpH����10.2 | ��Ʒ��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
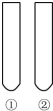 ���С��Լ����͡��Թ��е����ʡ�������ɡ�ʵ��Ŀ�ġ����ǣ�������
���С��Լ����͡��Թ��е����ʡ�������ɡ�ʵ��Ŀ�ġ����ǣ�������| ʵ��Ŀ�� | �Լ� | �Թ��е����� | |
| A | �ǻ��Ա����Ļ�����Ӱ�� | ������ˮ | �ٱ��ڱ�����Һ |
| B | ���Ա����Ļ�����Ӱ�� | ����KMnO4��Һ | �ٱ��ڼױ� |
| C | ��������û��̼̼˫�� | Br2��CCl2��Һ | �ٱ�����ϩ |
| D | ̼������Աȱ���ǿ | ʯ����Һ | �ٱ�����Һ��̼����Һ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
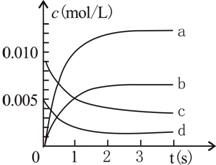 800��ʱ����2L�ܱ������ڼ���N0��02��������Ӧ��2N0��g��+O2��g��?2N02��g�������n��N0����ʱ��ı仯�������ش�
800��ʱ����2L�ܱ������ڼ���N0��02��������Ӧ��2N0��g��+O2��g��?2N02��g�������n��N0����ʱ��ı仯�������ش�| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO����mol�� | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
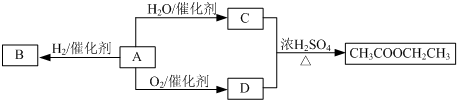
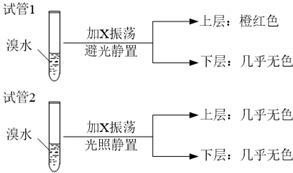
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com