
��7�֣��ס������صĵ缫���϶���������̼��������ͼ������ش��������⣺
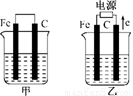
��1���������о�ʢ��CuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������ǣ��׳��е�________�����ҳ��е�________����
�����ҳ��������ĵ缫��Ӧʽ��________________��
��2���������о�ʢ�ű���NaCl��Һ����Ӧһ��ʱ���
��д���ҳ��з������ܷ�Ӧ�����ӷ���ʽ____________________��
�ڽ�ʪ��ĵ���KI��ֽ�����ҳظ�����������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2����������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
�����ҳ�ת��0��02 mol���Ӻ�ֹͣʵ�飬������Һ�������200 mL������Һ���Ⱥ��pH��________��
��1����̼��C��������Fe�� ��Cu2����2e��===Cu
��2����2Cl����2H2O2OH����H2����Cl2�� ��5Cl2��I2��6H2O===10HCl��2HIO3 ��13
���������׳�Ϊԭ��أ��ҳ�Ϊ���أ��Ҽ׳���FeΪ����������FeΪ������CΪ������
��1����ʢ��CuSO4��Һʱ���׳��з����ķ�ӦΪFe��Cu2��===Fe2����Cu���ҳ��з����ķ�ӦΪ2Cu2����2H2O2Cu��4H����O2����
��2�� �پ�ʢ��NaCl��Һʱ���׳��з���������ʴ���ҳ��з����ķ�ӦΪ2Cl����2H2OCl2����H2����2OH������Cl2������I2��������ʵ���֮��Ϊ5��1�����õ����غ㼴�ó�����ʽΪ5Cl2��I2��6H2O===10HCl��2HIO3����2Cl����2H2OCl2����H2����2OH�������ÿ����2 mol OH������ת��2 mol e��������n��OH������n��e������0��02
mol, c��OH������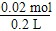 ��0��1 mol/L������pH��13��
��0��1 mol/L������pH��13��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1���������о�ʢ��CuSO4��Һ����Ӧһ��ʱ����к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
��2���������о�ʢ�б��͵�NaCl��Һ��
��д���׳��������ϵĵ缫��Ӧʽ��______________________________________________��
��д���׳���̿���ϵĵ缫��Ӧʽ��______________________________________________��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ��______________________________________________��
�����ҳ���ͨ��0.02 mol���Ӻ�ֹͣʵ�飬��ó�����Һ�������200 mL������Һ���Ⱥ��pHΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)�ס������صĵ缫���϶���������̼������ش��������⣺
(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________��
(2)�������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ________________________________________��
�ڼ׳���̿���ϵĵ缫��Ӧʽ��________���ҳ���̿���ϵĵ缫��Ӧ����________(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ��ĵ���KI��ֽ�����ҳ�̿��������������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2������������Ӧ��Cl2��I2���ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
�����ҳ�ת��0.02mol e����ֹͣʵ�飬������Һ�����200mL������Һ���Ⱥ��pH��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�ൺ���и�����ѧ��9�½��Լ�⻯ѧ�Ծ����������� ���ͣ������
��7�֣��ס������صĵ缫���϶���������̼��������ͼ������ش��������⣺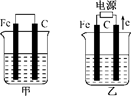
��1���������о�ʢ��CuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������ǣ��׳��е�________�����ҳ��е�________����
�����ҳ��������ĵ缫��Ӧʽ��________________��
��2���������о�ʢ�ű���NaCl��Һ����Ӧһ��ʱ���
��д���ҳ��з������ܷ�Ӧ�����ӷ���ʽ____________________��
�ڽ�ʪ��ĵ���KI��ֽ�����ҳظ�����������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2����������Ӧ��Cl2��I2�����ʵ���֮��Ϊ5��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ____________________��
�����ҳ�ת��0��02 mol���Ӻ�ֹͣʵ�飬������Һ�������200 mL������Һ���Ⱥ��pH��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ����У�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
��ͼ��ʾ���ס������صĵ缫���϶���������̼������ش��������⣺
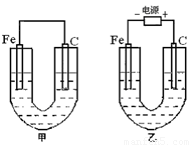
��1���ѻ�ѧ��ת��Ϊ���ܵ�װ���� ����ס����ҡ�����
��2�����������о�ʢ��CuSO4��Һ����Ӧһ��ʱ����к�ɫ�����������Ǽ׳��е� �����ҳ��е� ����
���ҳ���̼���ϵ缫��Ӧʽ�� ��
��3���������о�ʢ�б���NaCl��Һ��
��д���ҳ����ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
������ʪ��ĵ���KI��ֽ�����ҳ�̼��������������ֽ�� ����������������ӷ���ʽΪ �������ҳ��е����̪��Һ�� ��������̼�����������ֺ�ɫ��
�����ҳ���ͨ��0.02mol ���Ӻ�ֹͣʵ�飬��Ӧ�������Һ�����200mL����Һ���Ⱥ��c(OH-)Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��߿���ѧһ�ָ�ϰר���ۺϲ���6����ѧ��Ӧ�������仯���ս̰棩 ���ͣ������
(8��)�ס������صĵ缫���϶���������̼������ش��������⣺
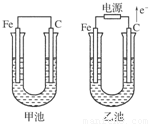
(1)�������о�ΪCuSO4��Һ����Ӧһ��ʱ���
���к�ɫ�����������Ǽ׳��е�________�����ҳ��е�________����
���ҳ��������ĵ缫��Ӧʽ��_______________________________________________��
(2)�������о�Ϊ����NaCl��Һ��
��д���ҳ����ܷ�Ӧ�����ӷ���ʽ________________________________________��
�ڼ׳���̿���ϵĵ缫��Ӧʽ��________���ҳ���̿���ϵĵ缫��Ӧ����________(�������Ӧ����ԭ��Ӧ��)��
�۽�ʪ��ĵ���KI��ֽ�����ҳ�̿��������������ֽ��������һ��ʱ����ַ�����ɫ��ȥ��������Ϊ������Cl2�����ɵ�I2������������Ӧ��Cl2��I2���ʵ���֮��Ϊ5 ��1�������������ᣬ�÷�Ӧ�Ļ�ѧ����ʽΪ
________________________________________________________________________��
�����ҳ�ת��0.02mol e����ֹͣʵ�飬������Һ�����200mL������Һ���Ⱥ��pH��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com