

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2O(H+) |
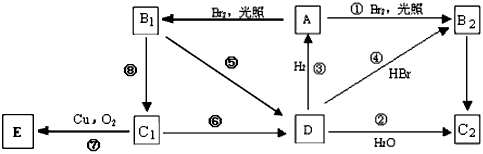
 ��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��
��������Ҫ�Ļ���ԭ�ϣ�����һ�������¿ɷ������±仯��







| ŨH2SO4 |
| �� |
| ŨH2SO4 |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
| ���� |

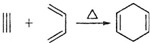
 ��R��R?������
��R��R?������







�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����Ҫ�ϳ�
�����Ҫ�ϳ�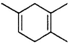 ���õ�ԭʼԭ�Ͽ�����
���õ�ԭʼԭ�Ͽ�����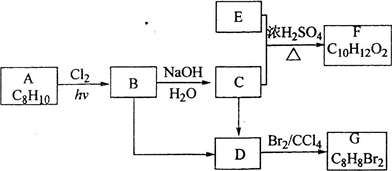
| NaOH |
| �� |
| NaOH |
| �� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������ɽ��������߿���ѧ����һ�֣� ���ͣ�058
��ѧ����1978���Ƶ�һ����A��A�ɿ�������B��������ԭ�ӱ���C��һ�ۻ�ȡ�����ã�A��Br2��CCl4��Һ����ɫ��A����ԭ�ӱ�һ����ԭ��ȡ��ֻ��һ�����ʣ�һ������C��ȫȼ�����õ�H2O��CO2�����ʵ���֮��Ϊ1.25��C���칹�岻����3�֣���C�Ķ������Ϊ3�֣�һ������B��ȫȼ�����ɵ�CO2��H2O�����ʵ���֮��Ϊ2��B����Է�����������26��С��78��
�Ը���������Ϣ�ƶ�A��B��C�Ļ�ѧʽ����д��A�Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��B�ķ���ʽΪ__________��
��2��д��C�Ľṹ��ʽ__________��
��3��A�ķ���ʽΪ__________��A�Ľṹ��ʽΪ__________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com