

 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğø£½ØŹ”ČżĆ÷ŹŠøßČż5ŌĀÖŹ¼ģĄķ×Ū»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
øõ”¢Ģś”¢Äų”¢ĶµČ½šŹō¼°Ęä»ÆŗĻĪļŌŚ¹¤ŅµÉĻÓŠÖŲŅŖÓĆĶ¾”£
£Ø1£©»łĢ¬øõŌ×ӵļŪµē×ÓÅŲ¼Ź½ĪŖ ”£
£Ø2£©CrO2Cl2ŗĶNaClO¾łæÉ×÷»Æ¹¤Éś²śµÄŃõ»Æ¼Į»ņĀČ»Æ¼Į”£ÖʱøCrO2Cl2µÄ·“Ó¦ĪŖ£ŗK2Cr2O2+3CCl4
2KC+2CrO2Cl2+3COCl2ӟӣ
¢ŁÉĻŹö·“Ó¦Ź½ÖŠ·Ē½šŹōŌŖĖŲµēøŗŠŌÓɓ󵽊”µÄĖ³ŠņŹĒ £ØÓĆŌŖĖŲ·ūŗűķŹ¾£©”£
¢Ś³£ĪĀŹ±CrO2Cl2ŹĒŅ»ÖÖŅ×ČÜÓŚCCl4µÄŅŗĢ壬Ōņ¹ĢĢ¬CrO2Cl2ŹōÓŚ ¾§Ģ唣
¢ŪCOCl2·Ö×ÓÖŠĖłÓŠŌ×Ó¾łĀś×ć8µē×Ó¹¹ŠĶ£¬COCl2·Ö×ÓÖŠ¦Ņ¼üŗĶ¦Š¼üµÄøöŹż±ČĪŖ ”£
£Ø3£©NiO”¢FeOµÄ¾§Ģå½į¹¹¾łÓėĀČ»ÆÄĘµÄ¾§Ģå½į¹¹ĻąĶ¬£¬ĘäÖŠNi2+ŗĶFe2+µÄĄė×Ó°ė¾¶·Ö±šĪŖ6.9”Į10£2nmŗĶ7.8”Į10£2nm”£ŌņČŪµć£ŗNiO FeO(Ģī”±<”±”¢ ”°=”±»ņ ”°>”±)”£
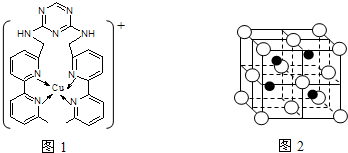
£Ø4£©CuClµÄŃĪĖįČÜŅŗÄÜĪüŹÕCOÉś³Éø“ŗĻĪļĀČ»ÆōŹ»łŃĒĶ[Cu2Cl2(CO)2”¤2H2O]£¬Ęä½į¹¹ČēĶ¼”£ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ £ØĢī±źŗÅ£©”£
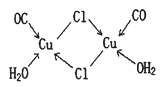
A£®øĆø“ŗĻĪļÖŠ“ęŌŚ»Æѧ½”ĄąŠĶÖ»ÓŠĄė×Ó¼ü”¢ÅäĪ»¼ü
B£®øĆø“ŗĻĪļÖŠClŌ×ÓµÄŌÓ»ÆĄąŠĶĪŖsp3
C£®øĆø“ŗĻĪļÖŠÖ»ÓŠCOŗĶH2O×÷ĪŖÅäĪ»Ģå
D£®COÓėN2µÄ¼Ūµē×Ó×ÜŹżĻąĶ¬£¬Ęä½į¹¹ĪŖC=O
£Ø5£©ĶłĮņĖįĶČÜŅŗÖŠ¼ÓČė¹żĮæ°±Ė®£¬æÉÉś³É[Cu(NH3)4]2+ÅäĄė×Ó”£ŅŃÖŖNF3ÓėNH3µÄæռ乹ŠĶ¶¼ŹĒČż½Ē׶ŠĪ£¬µ«NF3²»Ņ×ÓėCu2+ŠĪ³ÉÅäĄė×Ó£¬ĘäŌŅņŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğ½ĖÕŹ”ĖÕĪż³£ÕņĖÄŹŠøßČż½ĢѧĒéæöµ÷ŃŠ£Ø¶ž£©»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
Ķ”¢Ģ¼”¢µŖ”¢Įņ”¢ĀȵȏĒ×é³ÉĪļÖŹµÄÖŲŅŖŌŖĖŲ”£
£Ø1£©S”¢Cl×é³ÉµÄŅ»ÖÖ»ÆŗĻĪļµÄ·Ö×Ó½į¹¹ÓėH2O2ĻąĖĘ£¬Ōņ“Ė»ÆŗĻĪļµÄ½į¹¹Ź½ĪŖ ”£
N”¢O”¢SČżÖÖŌŖĖŲµÄµēøŗŠŌÓɓ󵽊”µÄĖ³ŠņĪŖ ”£
£Ø2£©ĶĄė×ÓŹĒČĖĢåÄŚ¶ąÖÖĆøµÄøØŅņ×Ó£¬ČĖ¹¤Ä£ÄāĆøŹĒµ±Ē°ŃŠ¾æµÄČČµć”£Ä³»ÆŗĻĪļY ÓėCu(¢ń)(¢ń±ķŹ¾»ÆŗĻ¼ŪĪŖ£«1)½įŗĻŠĪ³ÉĶ¼ĖłŹ¾µÄĄė×Ó£ŗ
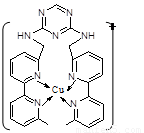
¢ŁŠ“³öCu(¢ń)µÄµē×ÓÅŲ¼Ź½ £»
¢ŚøĆĄė×ÓÖŠŗ¬ÓŠ»Æѧ¼üµÄĄąŠĶÓŠ £ØŃ”ĢīŠņŗÅ£©£»
A£®¼«ŠŌ¼ü B£®Ąė×Ó¼ü C£®·Ē¼«ŠŌ¼ü D£®ÅäĪ»¼ü
¢ŪøĆĄė×ÓÖŠCŌ×ÓµÄŌӻƷ½Ź½ÓŠ ”£
£Ø3£©ĻņĀČ»ÆĶČÜŅŗÖŠĶØČė×ćĮæµÄ¶žŃõ»ÆĮņ£¬Éś³É°×É«³ĮµķM£¬MµÄ½į¹¹ČēĶ¼ĖłŹ¾”£Š“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ ”£

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com