
·ÖĪö £Ø1£©øł¾ŻĮņĖįµÄÖŹĮæŹŲŗć¼ĘĖćĮņĖįŗĶĖ®µÄĢå»ż±Č£»
£Ø2£©ĀĢ·Æ£Ø FeSO4•7H2O £©ÖŠĮņµÄ»ÆŗĻ¼ŪĪŖ+6¼Ū£¬·“Ӧɜ³ÉĮĖ¶žŃõ»ÆĮņ£¬»ÆŗĻ¼Ū½µµĶĮĖ£¬øł¾ŻŃõ»Æ»¹Ō·“Ó¦µÄĢŲÕ÷æÉÖŖ£¬Ņ»¶ØÓŠŌŖĖŲµÄ»ÆŗĻ¼ŪÉżøߣ¬¾Ż“ĖÅŠ¶Ļ£»
£Ø3£©ĻČøł¾ŻĒāĘųµÄĢå»ż¼ĘĖćÉś³ÉĒāĘųŠčŅŖĢśµÄÖŹĮæ¼°Éś³ÉµÄ¶ž¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮ棬ŌŁøł¾Ż×ܼõÉŁµÄĢśµÄÖŹĮæ¼õČ„Éś³ÉĒāĘųŠčŅŖĢśµÄÖŹĮ漓ĪŖÓėČż¼ŪĢśĄė×Ó·“Ó¦ŠčŅŖĢśµÄÖŹĮ棬øł¾ŻÓėČż¼ŪĢśĄė×Ó·“Ó¦ĢśµÄÖŹĮæ¼ĘĖćČż¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮæŗĶÉś³É¶ž¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮ棬×ܶž¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮæ¼õČ„ÉĻŹö¶ž¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮ漓ĪŖŌČÜŅŗÖŠ¶ž¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮæ£¬Čż¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮæÓėŌČÜŅŗÖŠ¶ž¼ŪĢśĄė×ÓŗĶČż¼ŪĢśĄė×ÓÖ®ŗĶµÄ±ČÖµ¼“ĪŖŃõ»ÆĀŹ£»
£Ø4£©¢Ł²ÉÓĆ¼«ĻŽ·Ø¼ĘĖćÓė¹ĢĢå»ģŗĻĪļ·“Ó¦ŠčŅŖµÄøßĆĢĖį¼ŲµÄĪļÖŹµÄĮ棬Ź£ÓąµÄøßĆĢĖį¼ŲÓė£ØNH4£©2Fe£ØSO4£©2ČÜŅŗ·“Ó¦¼ĘĖćŠčŅŖµÄ£ØNH4£©2Fe£ØSO4£©2ČÜŅŗµÄĢå»ż£»
¢ŚĻČøł¾Ż£ØNH4£©2Fe£ØSO4£©2µÄĪļÖŹµÄĮæ¼ĘĖćÓėĘä·“Ó¦µÄøßĆĢĖį¼ŲµÄĪļÖŹµÄĮ棬Éč¹ĢĢå»ģŗĻĪļÖŠĮ½¹ĢĢåµÄÖŹĮ棬øł¾ŻĮ½¹ĢĢåµÄÖŹĮæ¼ĘĖćŠčŅŖµÄøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæ£¬Č»ŗóĮŠŹ½¼ĘĖć³öĮ½¹ĢĢåµÄÖŹĮ棬øł¾ŻÖŹĮæ·ÖŹż¹«Ź½¼ĘĖć¼“æÉ£®
½ā“š ½ā£ŗ£Ø1£©ÉčÅØĮņĖįµÄĢå»żĪŖxmL£¬Ė®µÄĢå»żĪŖymL£»Ļ”ŹĶĒ°ŗóĮņĖįµÄÖŹĮæ²»±ä£¬
ĖłŅŌ$\frac{1.84x”Į98%}{1.84x+1.0y}$£¬½āµĆx£ŗy=1£ŗ4.6£¬
¹Ź“š°øĪŖ£ŗ4.6£»
£Ø2£©ĀĢ·Æ£Ø FeSO4•7H2O £©ÖŠĮņµÄ»ÆŗĻ¼ŪĪŖ+6¼Ū£¬·“Ӧɜ³ÉĮĖ¶žŃõ»ÆĮņ£¬»ÆŗĻ¼Ū½µµĶĮĖ£¬øł¾ŻŃõ»Æ»¹Ō·“Ó¦µÄĢŲÕ÷æÉÖŖ£¬Ņ»¶ØÓŠŌŖĖŲµÄ»ÆŗĻ¼ŪÉżøߣ¬ĖłŅŌæÉŅŌÅŠ¶ĻÓŠĢśŌŖĖŲµÄ»ÆŗĻ¼ŪŌŚ·“Ó¦¹ż³ĢÖŠÉżøßĮĖ£¬
¹Ź“š°øĪŖ£ŗŹĒ£» ĀĢ·Æ·Ö½ā²śĪļÖŠÓŠSO2£¬ĮņŌŖĖŲ»ÆŗĻ¼Ū½µµĶ£¬±ŲÓŠŌŖĖŲ»ÆŗĻ¼ŪÉżøߣ»
£Ø3£©ÉčÓėĖį·“Ӧɜ³ÉĒāĘųŠčŅŖµÄĢśµÄÖŹĮæĪŖx£¬Éś³ÉµÄĮņĖįŃĒĢśµÄĪļÖŹµÄĮæĪŖy£¬
Fe+2H+=Fe 2++H2 ӟ
56g 1mol 22.4L
x y 0.224L
½āµĆx=0.56g£¬y=0.01mol£¬
ÓėFe3+·“Ó¦ŠčŅŖµÄĢśµÄÖŹĮæĪŖ5.00g-3.88g-0.56g=0.56g£¬
ÓėĢś·“Ó¦ŠčŅŖµÄČż¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮæĪŖm£¬Éś³ÉµÄ¶ž¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮæĪŖn£¬
Fe+2Fe3+=3Fe2+
56g 2mol 3mol
0.56g m n
½āµĆm=0.02mol£¬n=0.03mol£¬
ĖłŅŌŌĄ“ČÜŅŗÖŠ¶ž¼ŪĢśĄė×ÓµÄĪļÖŹµÄĮæĪŖ0.14mol-0.01mol-0.03mol=0.1mol£¬
ĖłŅŌŃõ»ÆĀŹĪŖ$\frac{0.02mol}{0.1mol+0.02mol}$£¬
¹Ź“š°øĪŖ£ŗ16.7%£»
£Ø4£©¢ŁøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖ£ŗ0.040L”Į0.150mol/L=0.006mol£¬
¼ŁÉč¹ĢĢå»ģŗĻĪļČ«²æĪŖCu2S£¬ŠčŅŖøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖx£¬
8MnO4-+5Cu2S+44H+=10Cu2++5SO2+8Mn2++22H2O
8 5
x $\frac{0.4g}{160g}$
x=0.004mol
Ź£ÓąµÄøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖ0.006mol-0.004mol=0.002mol£¬
0.002moløßĆĢĖį¼ŲŗĶ£ØNH4£©2Fe£ØSO4£©2ČÜŅŗĶźČ«·“Ó¦£®
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
1mol 5mol
0.002mol 1”Į10-3VL”Į0.2mol/L
½āµĆV=50£¬
¼ŁÉč¹ĢĢå»ģŗĻĪļČ«²æĪŖCuS£¬ŠčŅŖøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖy£¬
6MnO4-+5CuS+28H+=5Cu2++5SO2+6Mn2++14H2O
6 5
y $\frac{0.4g}{96g}$
y=0.005mol
Ź£ÓąµÄøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖ0.006mol-0.005mol=0.001mol£®
0.001moløßĆĢĖį¼ŲŗĶ£ØNH4£©2Fe£ØSO4£©2ČÜŅŗĶźČ«·“Ó¦£®
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
1mol 5mol
0.001mol 1”Į10-3VL”Į0.2mol/L
½āµĆV=25£¬
¹Ź“š°øĪŖ£ŗ25£¼V£¼50£»
¢ŚČōV=35£¬Óė35mL£ØNH4£©2Fe£ØSO4£©2ČÜŅŗĶźČ«·“Ó¦ŠčŅŖøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖ£ŗ
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O
1mol 5mol
0.014mol 0.035L”Į0.2mol/L
ĖłŅŌøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖ0.0014mol£¬
¹ŹÓė¹ĢĢå»ģŗĻĪļ·“Ó¦µÄøßĆĢĖį¼ŲµÄĪļÖŹµÄĮæĪŖ0.006mol-0.0014mol=0.0046mol£¬
ÉčCu2SµÄÖŹĮæĪŖxg£¬CuSµÄÖŹĮæĪŖyg£¬x+y=0.40g£®
8MnO4-+5Cu2S+44H+=10Cu2++5SO2+8Mn2++22H2O
8mol 800g
0.01xmol xg
6MnO4-+5CuS+28H+=5Cu2++5SO2+6Mn2++14H2O
6mol 480g
0.0125ymol yg
$\left\{\begin{array}{l}{x+y=0.4}\\{0.01xmol+0.0125ymol=0}\end{array}\right.$
½āµĆ$\left\{\begin{array}{l}{x=0.16\\;}\\{y=0.24}\end{array}\right.$
Ōņ»ģŗĻĪļÖŠCuSµÄÖŹĮæ·ÖŹż=$\frac{0.24g}{0.4g}$£¬
“š£ŗ»ģŗĻĪļÖŠCuSµÄÖŹĮæ·ÖŹżĪŖ60%£®
µćĘĄ ±¾Ģāæ¼²éĮĖøł¾Ż·½³ĢŹ½½ųŠŠÓŠ¹Ų¼ĘĖć£¬ÄŃ¶Č½Ļ“ó£¬×¢Ņā½ā“šĢÖĀŪĢāŹ±²ÉÓĆ¼«ĻŽ·Ø£¬øł¾Ż¼«ĻŽ·Ø¼ĘĖć³öĘäȔֵ·¶Ī§£®


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | 2£¬5-¶ž¼×»ł¼ŗĶé | B£® | 2£¬3-¶ž¼×»ł¶”Ķé | ||
| C£® | 2£¬3£¬4-Čż¼×»łĪģĶé | D£® | 2£¬2£¬3£¬3-Ėļ׻ł¶”Ķé |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
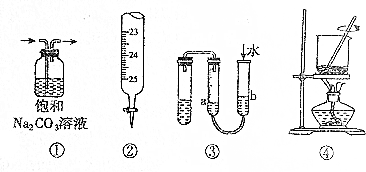
| A£® | ¢ŁÓĆÓŚ³żČ„CO2ÖŠµÄSO2 | B£® | ¢ŚÓĆÓŚĮæČ”20.00mL Na0HČÜŅŗ | ||
| C£® | ¢ŪÓĆÓŚÅŠ¶Ļ×°ÖƵÄĘųĆÜŠŌ | D£® | ¢ÜÓĆÓŚ½«ŗ£“ųČ¼ÉճɻŅ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ijČÜŅŗÓėNa0HČÜŅŗ¹²ČČ£®²śÉśŹ¹ŹŖČóµÄŹÆČļŹŌÖ½±äŗģµÄĘųĢ壬ĖµĆ÷ŌČÜŅŗÖŠ“ęŌŚNH${\;}_{4}^{+}$ | |
| B£® | ijČÜŅŗÖŠ¼ÓČĖAgN03ČÜŅŗŹ±£¬²śÉś°×É«³Įµķ£®ĖµĆ÷ŌČÜŅŗÖŠŗ¬ÓŠCl- | |
| C£® | ÓĆ²¬ĖæÕŗČ”Ä³ČÜŅŗŌŚ¾Ę¾«µĘ»šŃęÉĻ×ĘÉÕŹ±£¬»šŃę³Ź»ĘÉ«£¬ĖµĆ÷ŌČÜŅŗÖŠŗ¬ÓŠ½šŹōÄĘ | |
| D£® | ijČÜŅŗÖŠ¼ÓČĖBaCl2ČÜŅŗŹ±£¬²śÉś°×É«³Įµķ£®ŌČÜŅŗÖŠæÉÄÜ“ęŌŚAg+»ņSO42-»ņCO32- |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ŃĪĖįµÄĪļÖŹµÄĮæÅØ¶ČŹĒ0.5mol•L-1 | B£® | ĒāŃõ»ÆÄĘČÜŅŗµÄĢå»żŹĒ250mL | ||
| C£® | ĢśŗĶ¹čµÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ1 | D£® | ĢśŗĶ¹čµÄÖŹĮæÖ®±ČĪŖ1£ŗ2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā

| A£® | aÓėb±Č½Ļ£¬bŹ¹ÓĆĮĖ“߻ƼĮ | B£® | aÓėb±Č½Ļ£¬bĪĀ¶Čøüøß | ||
| C£® | aÓėb±Č½Ļ£¬bµÄŃ¹Ēæøü“ó | D£® | aÓėb±Č½Ļ£¬b·“Ó¦ĖŁĀŹøü“ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
| Ź±¼ämin | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | w.9 |
| n£ØSO2£© | 2.00 | 1.92 | 1.84 | 1.76 | 1.76 | 1.64 | 1.52 | 1.40 | 1.40 | 1.40 |
| n£ØO2£© | 1.00 | 0.96 | 0.92 | 0.88 | 0.88 | 0.82 | 0.76 | 0.70 | 0.70 | 0.70 |
| n£ØSO3£© | w0 | 0.08 | 0.16 | 0.24 | 0.24 | 0.36 | 0.48 | 0.60 | 0.60 | 0.60 |
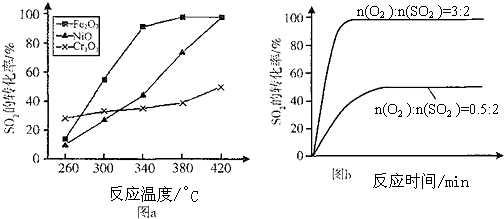
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com