

| ”÷ |
| ”÷ |


| ”÷ |
| ”÷ |
 £¬
£¬



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠĪ÷³ĒĒųø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
(14·Ö) ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ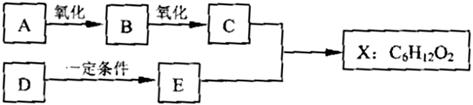
Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©AµÄĆū³ĘŹĒ ”£
£Ø2£©BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½(²»ŗ¬A)£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
£Ø6£©ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģŗÓÄĻŹ”ø߶žĻĀµŚČż“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖ
DŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»ī
ÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā”£
(1)AµÄĆū³ĘŹĒ ”£
(2)BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
(3)C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
(4)Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ (²»ŗ¬A)£ŗ ”£
(5)XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ ”£
(6)ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģ±±¾©µŚ66֊ѧø߶žĻĀѧʌµŚ¶ž“ĪŌĀæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø14·Ö£©ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©A·Ö×ÓµÄĆū³ĘŹĒ ”£
£Ø2£©B·Ö×ÓÖŠĖłŗ¬¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E”śXµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ ·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½£Ø²»ŗ¬A£©£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010Äź±±¾©ŹŠĪ÷³ĒĒųø߶žĻĀŃ§ĘŚĘŚÄ©æ¼ŹŌ»ÆѧŹŌĢā ĢāŠĶ£ŗĢīæÕĢā
(14·Ö) ĻĀĶ¼ÖŠXŹĒĪŽÖ§Į“µÄ”¢¾ßÓŠ¹ūĻćĪ¶µÄŗĻ³ÉĻćĮĻ£¬æÉÓĆÓŚµ÷Å䶹ÖÖ¹ūĻćŠĶĻć¾«”£ŅŃÖŖDŌŚ±ź×¼×“æöĻĀµÄĆܶČĪŖ1.25 g/L£¬Ęä²śĮææÉŅŌÓĆĄ“ŗāĮæŅ»øö¹ś¼ŅŹÆÓĶ»Æ¹¤·¢Õ¹Ė®Ę½”£EŹĒÉś»īÖŠ³£¼ūµÄŅ»ÖÖÓŠ»śĪļ”£ø÷ĪļÖŹ¼ä×Ŗ»Æ¹ŲĻµČēĻĀ£ŗ

Ēė»Ų“šĻĀĮŠĪŹĢā”£
£Ø1£©AµÄĆū³ĘŹĒ ”£
£Ø2£©BÖŠĖłŗ¬µÄ¹ŁÄÜĶÅŹĒ ”£
£Ø3£©C+E XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ
·“Ó¦”£
XµÄ»Æѧ·“Ó¦ĄąŠĶŹĒ
·“Ó¦”£
£Ø4£©Š“³öČĪŅāĮ½ÖÖÓėA¾ßÓŠĻąĶ¬¹ŁÄÜĶŵÄAµÄĶ¬·ÖŅģ¹¹ĢåµÄ½į¹¹¼ņŹ½ (²»ŗ¬A)£ŗ
Ӣ ӣ
£Ø5£©XÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
£Ø6£©ŅŌDĪŖŌĮĻÉś²śŅ»ÖÖ³£¼ūĖÜĮĻµÄ»Æѧ·½³ĢŹ½ŹĒ
ӣ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com